Understanding Aldehydes and Ketones in Organic Chemistry
This chapter discusses carbonyl compounds focusing on aldehydes and ketones. It covers their structural differences, drawing, nomenclature, physical properties, synthesis methods, and nucleophilic attack reactions. Additionally, it includes common classes of carbonyl compounds, naming conventions for aldehydes, substituent locations, and IUPAC nomenclature guidelines.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
ALDEHYDES AND KETONES Dr. Shatha I Alaqeel 108 Chem
2 Learning Objectives Chapter eight discusses carbonyl compounds and by the end of this chapter the students will: Know the structural differences between aldehydes and ketones Know how to draw aldehydes and ketones know the common and IUPAC nomenclature of aldehydes and ketones Know the physical properties of aldehydes and ketones Know how to synthesize an aldehyde or a ketone. Know the different nucleophilic attack reactions at the carbonyl carbon. 108 Chem
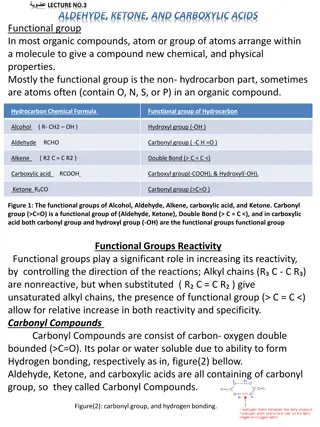
3 ALDEHYDES: STRUCTURE AND NOMENCLATURE Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, C=O. The carbon atom of this group has two remaining bonds that may be occupied by hydrogen or alkyl or aryl substituents. If at least one of these substituents is hydrogen, the compound is an aldehydeRCHO or RCH=O. If neither is hydrogen, the compound is a ketoneRCOR` (R and R`=alkyl or aryl). 108 Chem
4 Some Common Classes Carbonyl Compounds 108 Chem
5 Common Names of Aldehydes In the common system, aldehydes are named from the common names of the corresponding carboxylic acid. The ic acid ending is replaced with aldehyde . The aldehyde group is always at the end of a chain (terminal). Structure IUPAC name Common name Structure IUPAC Common name HCO2H CH3CO2H CH3CH2CO2H CH3(CH2)2CO2H CH3(CH2)3CO2H CH3(CH2)4CO2H methanoic acid ethanoic acid propanoic acid butanoic acid pentanoic acid hexanoic acid HCHO CH3CHO CH3CH2CHO CH3(CH2)2CHO CH3(CH2)3CHO CH3(CH2)4CHO methanal ethanal propanal butanal pentanal hexanal formic acid acetic acid propionic acid butyric acid valeric acid caproic acid formaldehyde acetaldehyde propionaldehyde butyraldehyde valeraldehyde caproaldehyde 108 Chem
6 Substituents locations are given using Greek letters ( , , , , , .) beginning with the carbon next to the carbonyl carbon, the -carbon. O OH O O H CH2C CH3CHBrCH2C H CH3CHCH2CH2C H -bromobutyraldehyde -hydroxyvaleraldehyde -phenylacetaldehyde Aromatic aldehydes are usually designated as derivatives of the simplest aromatic aldehyde, Benzaldehyde OH O O O O H H H H H3CO O2N Benzaldehyde p-Nitrobenzaldehyde o-Hydroxybenzaldehyde p-Methoxybenzaldehyde Salicylaldehyde Anisaldehyde 108 Chem
7 IUPAC Nomenclature of Aldehydes Select the longest continuous carbon chain that contains the C=O group and replace the ending -e by the suffix -al The CHO group is assigned the number 1 position and takes precedence over other functional groups that may the present such as OH, C=C If the CHO group is bonded to a ring, name the ring and add the suffix -carbaldehyde. 108 Chem
8 O OH O O H CH2C CH3CHBrCH2C H CH3CHCH2CH2C H 3-bromobutanal 4-hydroxypentanal 2-phenylethanal O O O Cl H O C H H3 C CH H H3CHC=HC C H 3-Hydroxypropanal 2-Chloropropanal 2-Butenal O O HO CHO H H C C 3-hydroxycyclopentanecarbaldehyde benzenecarbaldehyde cyclohexanecarbaldehyde 108 Chem
9 Nomenclature of Ketones Common name: listing the alkyl substituents attached to the carbonyl group alphabetically, followed by the word ketone. As with aldehydes, substituents locations are given in common names using Greek letters ( , , , , , .) beginning with the -carbon. IUPAC system: Find the longest chain containing the carbonyl group, and change the -e ending of the parent alkane to the suffix -one. Number the carbon chain to give the carbonyl carbon the lower number. Apply all of the other usual rules of nomenclature. Ketones are just below aldehydes in nomenclature priority. A ketone group is named as an oxo substituent in an aldehyde. 108 Chem
10 O O O O H3 C C CH3 H3 C C C6H5 H3 C C CH=CH2 H5C6 C C6H5 Common Dimethyl ketone Methyl phenyl ketone Methyl vinyl ketone Diphenyl ketone Acetone Acetophenone Benzophenone IUPAC Propanone Phenyl ethanone 3-Buten-2-one Diphenylmethanone O O OCH3 CH3 O CH3CHCCHCH3 Cl C CH2CH2CH2 CH3CCH2CHCH3 CH3 Methyl isobutyl ketone (MIBK) -Methoxypropyl phenyl ketone -Chloroethyl isopropyl ketone O O O OH CHO C2H5 C C2H5 Cyclopentylpropanone 3-Ethyl-2-hydroxycyclohexanone 5-Oxohexanal 108 Chem
11 PHYSICAL PROPERTIES OF KETONES AND ALDEHYDE + O C C+ O- C O C O + Aldehydes and ketones are polar compounds, Because the polarity of the carbonyl group. Polarization of CO group creates Dipole-dipole attractions between the molecules of aldehydes and ketones, resulting in higher boiling points than nonpolar alkanes and ether. aldehydes and ketones lower than alcohols Because Dipole- dipole attractions , are not as strong as interactions due to hydrogen bonding. The lower aldehydes and ketones are soluble. Acetone, formaldehyde and acetaldehyde are miscible in water. O + + C O H H O C 108 Chem
12 Preparation of Aldehydes and Ketones Oxidation of alcohols Cu / heat HCHO CH3OH aldehyde CrO3 / Pyridine CH3CH2OH CH3CHO O OH K2Cr2O7 / H+ Ozonolysis of alkenes H i) O3 ii) Zn /H2O O O+ 108 Chem
13 Hydration of alkynes O CH3 H / H2SO4, HgSO4 H-OH HC C CH3 C C H3C C CH3 O H H Acetone Propyne (an unstable enol) (a stable carbonyl compound) Friedel Grafts acylation O O AlCl3 +R R Cl 108 Chem
14 REACTIONS OF ALDEHYDES AND KETONES Nucleophilic Addition Reaction to the carbon-oxygen double bond. tetrahedral alkoxide Nu:- : .. : : O : : O : O C R sp3 C + R sp2 R H C Nu:- E+ Nu 1-Reduction of carbonyl group Addition of metal hydrides: Formation of alcohols. 108 Chem
15 Reduction by hydride reagents, Lithium aluminium hydride LiAlH4 or Sodium boron hydride NaBH4. 1) LiAlH4 / dry ether or NaBH4 2) H3O+ 1) LiAlH4 / dry ether or NaBH4 2) H3O+ 1) LiAlH4 / dry ether or NaBH4 2) H3O+ OH 2H2 / Pt O 1) NaBH4 2) H2O OH 108 Chem
16 2- Addition of Grignard Reagents: Formation of alcohols R` O 1) Dry ether 2) H3O+ R`MgX + R R CH OH H O OH 1) Dry ether 2) H3O+ R``MgX + R R C R`` R` R` 108 Chem
17 3-Oxidation reaction A) R-CHO KMnO4 or K2Cr2O7 ArCOOH RCOOH or Ar-CHO or B) Iodoform reaction: The reaction occurs in any aldehyde or ketone containing CH3CO. ONa +3 NaI +3 I2 +CHI3 +4 NaOH R O R O I2 / NaOH CHI3 COONa + O 108 Chem
18 4- Addition of Hydrogen Cyanide: Formation of cynohydrins R +HCN R Cyanohydrine `R OH `R O CN CN CH2NH2 H2 / Pt OH O +HCN OH or LiAlH4 and H3O+ Benzaldehyde cyanohydrine COOH CN O H3O+ OH OH +HCN heat 5-Addition of acetylide ions: R R H3O+ + `R R`` ``R CNa `R O OH OH O H3O+ + CNa 108 Chem
19 6- Addition of alcohols: R R R R```OH H+ + `R OR`` `R OR`` R``OH `R O H+ OH OR``` R` = H Aldehyde Hemiacetal Acetal R` = alkyl Ketone Hemiketal Ketal OC2H5 OH H+ C2H5OH C2H5OH + O OC2H5 OC2H5 H+ Hemiacetal Acetal OC2H5 H+ OH C2H5OH + C2H5OH O OC2H5 OC2H5 H+ HemiKetal Hemiacetal (new) Ketal Acetal (new) 108 Chem
20 7- Addition of Ammonia and Ammonia Derivatives NH NH3 Imine OH NH2OH Hydroxyamine N Oxime O NH2 H2N-NH2 N Hydrazone Hydrazine 108 Chem
21 Thank You for your kind attention ! Thank You for your kind attention ! Questions Questions 108 Chem