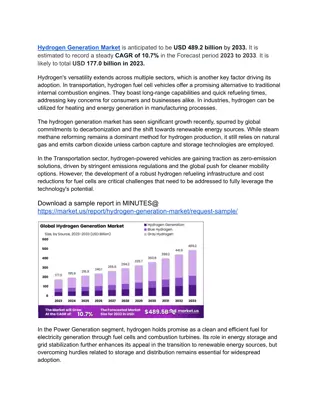
Overview of Hydrogen: Properties, Isotopes, and Characteristics
Hydrogen, a colorless gas with atomic number 1 and mass 1.008 amu, exhibits electropositive and electronegative characteristics due to its electron configuration. It has three isotopes - protium, deuterium, and tritium - with varying reaction rates. Protium is the most abundant isotope. This element's nucleus contains one proton and no neutron, making it unique among elements. Hydrogen's properties, such as oxidation state, electron configuration, and isotopic variations, contribute to its versatile applications in various industries.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Hydrogen By Dr. Atul Kumar Singh Assistant Professor Department of Chemistry M. L. Arya College, Kasba Purnia -854330 India
Hydrogen Symbol: H Group: 1 Period: 1 Atomic number: 1 Atomic Mass: 1.008 amu Appearance : Colorless gas Electronic Configuration :1s1
Oxidation state: -1, +1 Electronegativity: Pauling scale 2.20 Ionisation energies: 1st 1312.0 kJ/mol Covalent radius: 31 5 pm Van der waals radius: 120 pm Melting point: (H2) -259.16 C (-434.49 F) Boiling point: (H2) -252.879 C (-423.182 F)
Nucleus of the hydrogen contains one proton and zero neutron i.e. the neutrons absent in hydrogen nucleus. Hydrogen contains one electron arranged in the s orbital. The s orbital of hydrogen contain only one electron so it has a tendency to either lose this electron or gain this electron.
Electropositive Character of Hydrogen: The s orbital of hydrogen contain only one electron so it has a tendency to lose this electron like alkali metal thus has an electropositive character. Electronegative Character of Hydrogen: The s orbital of hydrogen contain only one electron so it has a tendency to gain an electron to complete 1s subshell like the halogens thus has an electronegative character.
Isotopes of Hydrogen Hydrogen has three isotopes (protium, deuterium and tritium) All three isotopes have same chemical properties but different rates of reactions. a) Protium or ordinary Hydrogen: Most common and constitutes 99.984 % of total hydrogen.
b) Deuterium or Heavy Hydrogen: Constitutes only 0.016 % of total hydrogen. c) Tritium: Constitutes only 10-15 % of total hydrogen. Formed in upper atmosphere by nuclear reaction.