Understanding Covalent Bonding in Chemistry
Explore the concept of covalent bonding in chemistry, where atoms share electrons through orbital overlap to form stable molecules. Learn about why covalent bonds exist, how bond length affects the stability of a molecule, the model for covalent bonding, Lewis structures, and the characteristics of multiple bonds. Discover the key principles and structures involved in covalent bonding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
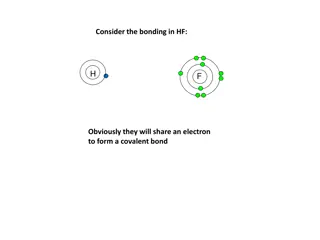
Covalent Bond Chemical bond formed by the sharing of a pair of electrons
Orbital Overlap Shared pairs are actually orbitals of two atoms overlapping
Why Covalent Bonds Exist Atoms share electrons when their orbitals overlap When orbitals overlap, electrons are simultaneously attracted to both nuclei
Bond Length As atoms come closer, increasing electron density results in a decrease in potential energy of system As atoms become very close, electrostatic repulsion between nuclei increases Bond distance is a compromise between increased overlap drawing them together and increased repulsion pushing them apart There is an optimal bond distance where energy is lowest
Model for Covalent Bonding Localized electron model molecule is composed of atoms that are bound together by sharing pairs of electrons Pairs of electrons localized on one atom are called Lone Pairs Electrons found in the space between atoms are called Bonding Pairs
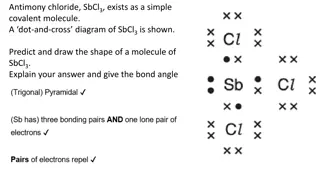
Lewis Structure Show covalent bonds between atoms Dots used to represent unshared pairs of electrons Dash used to show electron pair shared in a bond Cl2 H2
Multiple Bonds Single Bond shares 1 pair of electrons Double Bond shares 2 pair Triple Bond shares 3 pair As the number of shared pairs increases, the distance between the bonded atoms decreases
Drawing the Lewis Structure Sum up all valence electrons Write symbols for atoms involved so as to show which atoms are connected Draw a single bond between each atom Complete octets on outer atoms Place leftover electrons on the central atom Use multiple bonds if central atom does not receive an octet
Draw PCl3 HCN ClO3- NO+