Understanding Atomic Properties and Covalent Radii in Chemistry
Exploring the concept of atomic properties including the sizes of atoms and ions, the three common operational radius concepts (covalent, crystal, and van der Waals), and the calculation of covalent radius for homonuclear and heteronuclear diatomic molecules. This overview delves into the significance of bond lengths in molecules and the methods used to determine atomic radii in chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Periodic Table Course Instructor: Dr. Atul Kumar Singh Assistant Professor Department of Chemistry M. L. Arya College, Kasba Purnia -854330 India
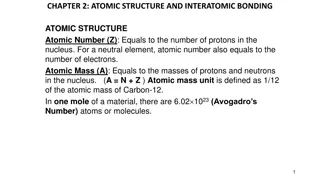
Atomic properties Size of atoms and ions Generally the sizes of atoms or ions is the magnitude of their respective radii. The radius of the atom or ion is the distance between the centre of the nucleus and the outermost shell of electrons. However, the absolute size of atom or ion is difficult to define because according to quantum mechanics there is no certainty in position of electron
at any time and it is not possible to get a isolated atoms. There are three operational concept of radius are commonly used (a) covalent radius (b) crystal radius (c) van der Waals' radius.
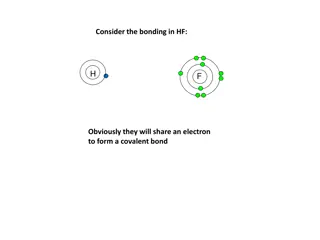
1. Covalent Radius Covalent radius is defined as one of the half distance between two nuclei of two atoms (same atoms) bonded together by a single covalent bond. Distance can be measured by X-ray diffraction or spectroscopic studies. Covalent radius is expressed in term of picometers (pm ) or angstroms ( )
Covalent radius for homonuclear diatomic molecule Can be calculated as: For example, spectroscopic data show that distance between the nuclei of two hydrogen atom is 0.74 so the covalent radius of hydrogen atom is 0.37 (half of the distance between the nuclei of two hydrogen atom )
Bond length of Cl2 (d Cl-Cl) is1.98 hence For hetero-nuclear diatomic AB molecule (linked by a single covalent bond and have nearly same electronegativity) the bond length dA- B is equal to sum of covalent radius of the two atoms.
Bond length of CH4 (internuclear distance between C and H) (d C-H) is 1.14 and Covalent radius of carbon is 0.77 then the covalent radius of hydrogen can be calculated as
For hetero-nuclear diatomic molecule AB (linked by a single covalent bond and have large difference in the electronegativity of two elements A and B, then the covalent radius calculated by using following equation.
Most of the metal do not form covalent compound except covalent hyride and organometallic compounds, it is therefore not possible to determine the covalent radii of metals. Atomic volumes of metals atoms can be easily determined and is known as metallic radii.