Understanding the Lewis Octet Rule in Chemical Bonding
Exploring the concept of shared electron bonds focusing on valence electrons, core electrons, Lewis dot structures, and the Lewis Octet Rule. Learn how to apply these rules to build organic compounds and understand the stability of elements through visual representations.
Uploaded on Sep 20, 2024 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
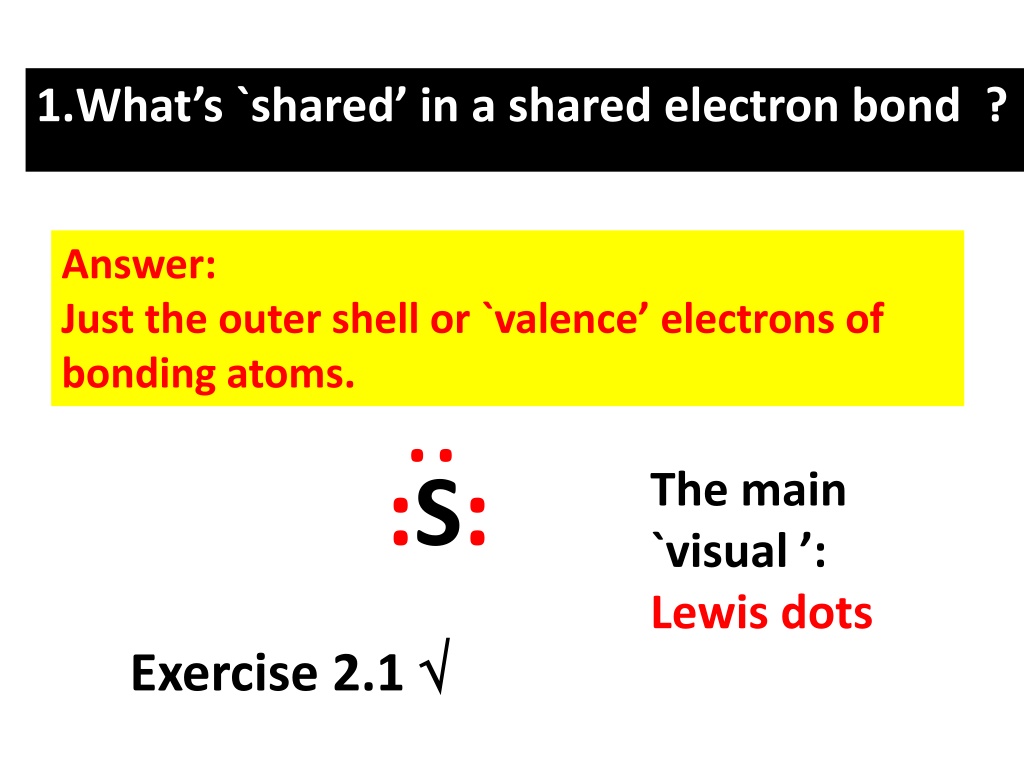
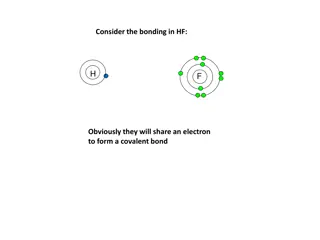
1.Whats `shared in a shared electron bond ? Answer: Just the outer shell or `valence electrons of bonding atoms. .. The main `visual : Lewis dots :S: Exercise 2.1
Valence vs `Core electrons How many `core electrons does S have ? 10 (Ne) Inert gas core electrons for S How many total electrons does S have ?16 How many valence electrons does S have ? .. Valence shell or outer shell of S 6 1 2 3 4 5 6 :S: . Dot picture shows only valence electrons
2. What rules govern the number of bonds to the elements and how do we use these rules to build organic (and other non-ionic) compounds ? Uncle `Gil sez: If you can count to 8, even you can do it, maggot .. Gilbert Newton Lewis: (Uncle Gil) American Chemist
Foundations of the `Lewis Octet Rule What we learned From chapter 2: e- Na+ Cl- + Na + Cl (+) and (-)attracted to each other to form ionic bond 11 e- 10 e- 17 e- 18 e- Ar Ne like Ne Like Ar # valence electrons ? 8 8 .. DOT PICTURE VISUAL Lewis `octet is the most stable form of elements all atoms (X) want to look like this,whether ionic or not ionic : [X]: ..
USING THE `OCTET RULE How to build a covalent compound using `Lewis Rules (same process as on pp 102-106 of text. The Lewis Octet Rule 1) Count all the valence electrons on all the bonding elements Ex. CO2 Valence e- on C = 1 x 4 = 4 Valence e- on 2O = 2 x 6 = 12 total valence e- = 4+12= 16
How to build a covalent compound using `Lewis Rules (same process as on pp 102-106 of text) the Lewis Octet Rule (continued) 2)Start by drawing single bonds to each atom and compute the total number of electrons in those. Electrons in bonds= 2 bonds x 2 e-/bonds = 4 Ex. O-C-O
How to build a covalent compound using `Lewis Rules (same process as on pp 102-106 of text) .continued 3) Distribute the remaining valence electrons not in bonds one by one and evenly to each element in the molecule. If an element reaches an `octet , stop placing electrons on it and distribute what s left to the other elements. Electrons not in bonds = 16-4=12 Distribute them evenly among C and O Ex. O-C-O
How to build a covalent compound using `Lewis Rules (same process as on pp 102-106 of text) .continued 4) Check each atom for an octet. If all have octets, you have the right Lewis structure. If not, go back to step 2, include a double bond somewhere and repeat steps 2-4 until a complete octet is achieved around each element assuming the given electron count Ex. O-C-O x x
Step 2 repeat: add another bond x Electrons in bonds = 3 bonds x 2 e-/bonds = 6 O=C-O Skip C now has octet Electrons not in bonds = 16-6 =10 Step 3 repeat: distribute 10 non-bonded electrons evenly Step 4 repeat: check to see if every element has an octet
Back to Step 2 repeat: add another bond x Electrons in bonds = 3 bonds x 2 e-/bonds = 6 O=C-O Skip C now has octet Electrons not in bonds = 16-6 =10 Step 3 repeat: distribute 10 non-bonded electrons evenly (into lone pairs) Step 4 repeat: check to see if every element has an octet
Step 2 repeat repeat: add yet another bond Electrons in bonds = 4 bonds x 2 e-/bonds = 8 Skip C..already has octet O=C=O Electrons not in bonds = 16-8 =8 Step 3 repeat repeat: distribute 8 non-bonded electrons evenly (into lone pairs) Step 4 repeat repeat: check to see if every element has an octet
LEWIS PREDICTION FOR BONDING IN CO2 Lone pair (2 e- per pair): 2 dots = one pair O=C=O Bond pairs (2 e-/bond): one line = 1 bond Some helpful Lewis language: bond pairs vs lone pairs
2) What rules govern the number of bonds to the elements and how do we use these rules to build organic (and other non-ionic) compounds ? Lewis s Answer: 2. LEWIS (OCTET) RULE
EXERCISE 2. PLAYING THE LEWIS ELECTRON DOT GAME Mole $$ !!!!
??? Doc s annoying question habit rears it ugly head again .
I am so annoying ??? How do we know what `hooks to what in more complicated molecules ? Examples where confusion can arise: CO2 C-O-O or O-C-O ?? SO2 S-O-O or O-S-O ?? C O O about rings ? What S O O
CO2 SO2 C-O-O or O-C-O ?? S-O-O or O-S-O ?? Simple rule of thumb #1: Elements closer to the center of the Periodic Table tend to be in the center of a molecule
Applying Rule of Thumb 1 to CO2 C is closer to center line=> C in center of CO2C-O-O or O-C-O ?? `center of Table `center line
Applying Rule of Thumb 1 to SO2 S-O-O or O-S-O ?? S is closer to center =>S in center of SO2
What about rings ? Simple rule of thumb #2: C S O O O O Compound structures tend to minimize `strain and maximize bond angles
Docs questions revisited 1.What s `shared in a shared electron bond ? Valence electrons only; 2 per bond 2.What rules govern the number of bonds to the elements and how do we use these rules to build organic (and other non-ionic) compounds ? Lewis Octet Rule + Rules of Thumb 1 and 2 3.How do we `read organic compound formulas and deduce their bond and electron arrangements ? ???
The simple as 1,2,3,4 HONC bonding rules (for organic compounds only) Element bond count to element lone pairs on element H 1 O 2 N 3 C 4 O, N & C BOND COUNT + LONE PAIR COUNT = 4 0 2 1 0 *USE FOR EXERCISE 2.4
Simple Bonding Rules for Organic* compounds: the HONC Rules *Compounds made of C+ H with options to include O and N EXAMPLE #1: ethane (C2H6) = one of the `natural gases H H H C C H H H
EXAMPLE #2: ethanol (C2H6O) =drinking alcohol H H H C C OH H H
EXAMPLE #3: aspirin (C10H12O5 ) = what to take after excess alcohol H OH O C C H H O C H O C C C H C C H C H H C H H H
EXAMPLE #4: glycine (C2H5NO2) =amino acid, building block of proteins (building block of all living things ) H H N H C O H C H O
EXAMPLE #5: DNA (BLUEPRINT for all living things) Deoxyribonucleic acid
POPULAR GRAPHIC FOR DNA
organic chemical notation (see also pp 108-109 of text) 3 different ways to read/draw HONC structures 1) Complete skeletal form H OH H Rubbing alcohol H C C C H H H H All or most all lone pairs, bonds and atoms shown explicitly.
Aside on organic chemical notation (see also pp 108-109 of text) 3 Different ways to read/draw HONC structures (continued) 2) Condensed form OH H OH H H C C C H H3C C CH3 H H H Complete skeletal form H Methyl (CH3) and methylene groups (CH2) written without C-H bonds, but lone pairs still shown.
Aside on organic chemical notation (see also pp 108-109 of text) 3 Different ways to read/draw HONC structures (continued) 3) Abbreviated bond line form (most used by organic chemists) H H OH Complete Skeletal form OH H C C C H H H H All kinks , ends and crossings are C. If no other groups showing,assume H around C to reach 4 bonds to C. Lone pairs assumed via HONC rules(though text doesn t.)
Why bond line form is preferred by organic chemists SIMPLER LOOKING IS PRETTIER OH aspirin O C OH O O O C H H C C C O O C C C H H H C H H Bond line form Complete skeletal form messy,ugly I m neat & pretty
+ more board practice : complete skeletal bond line condensed condensed complete skeletal bond line