Compositional Analysis of Rock-Forming Minerals Using Triangular Diagrams
Geologists frequently utilize triangular diagrams to determine mineral compositions in the Al2O3-SiO2-K2O system. This analysis helps identify stable minerals under Earth-surface conditions, considering primary weathering minerals. The process involves calculating mole percentages of oxides in minerals like microcline and K2O, aiding in understanding phase compositions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
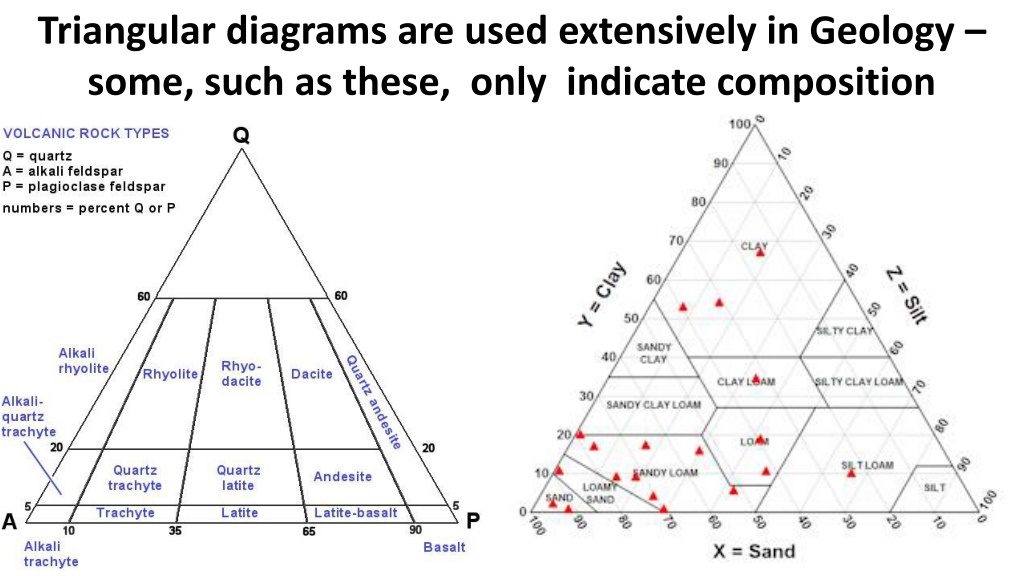
Triangular diagrams are used extensively in Geology some, such as these, only indicate composition
We will use Triangular Diagrams to determine the stable minerals that will go on an activity diagram. Assume ubiquitous water so H2O is used to balance equations. Determine what minerals to consider under Earth- surface conditions in the Al2O3-SiO2-K2O system. Include some common primary minerals that are weathering. Don t worry about SiO2 (am) Any rock-forming minerals w/ just K2O & H2O? Any rock-forming minerals w/ just Al2O3 & H2O? Al2O3 Al (OH)3 Al O (OH) corundum gibbsite boehmite No These are only a subset of the possible minerals.
Any rock-forming minerals w/ just K2O & SiO2? Any rock-forming minerals w/ just Al2O3 & K2O? No Any rock-forming minerals w/ just Al2O3 & SiO2? kaolinite Al2 Si2 O5 (OH)4 Al2 Si4 O10 (OH)2 Al2 SiO5 Any rock-forming minerals w/ K2O, Al2O3 & SiO2? microcline KAlSi3O8 KAl3Si3O10(OH)2 These are only a subset of the possible minerals. No kyanite pyrophyllite These are only a subset of the possible minerals. muscovite leucite KAlSi2O6 kaliophilite KAlSiO4 Now we need Gf for these minerals
Gf Kj/mol -322.1 -379.1 -856.6 -1582.3 -1155.1 -915.8 -3799.7 -5266.1 -2005.3 -2871.4 -3742.9 -5608.4 -2443.9 -237.1 (Number of moles) Al2O3 Formula K2O SiO2 Compound K-Oxide K-Hydroxide Quartz Corundum Gibbsite Boehmite Kaolinite Pyrophyllite Kaliophite Leucite Microcline Muscovite Kyanite H2O K2O KOH SiO2 Al2O3 Al (OH)3 Al O (OH) Al2 Si2 O5 (OH)4 Al2 Si4 O10 (OH)2 KAlSiO4 KAlSi2O6 KAlSi3O8 KAl3Si3O10(OH)2 Al2SiO5 water
Compose a table showing the compositions of phases. Start by calculating the number of moles of each of the three oxides in the mineral and then convert to mole %. For practice, calculate values for K2O and microcline. K2O Al2O3 SiO2 Formula K2O Compound K-oxide Practice calculating mole percents so you know how to do it for your problem set. 1 100% 0.5 0.5 3 12.50%Completed table is on the next slide. # of moles and mol percent 12.50% 75% 0% 0% 0 0 KAlSi3O8 Microcline
Gf kJ/mol -322.1 -379.1 -856.6 -1582.3 -1155.1 -915.8 -3799.7 -5266.1 -2005.3 -2871.4 -3742.9 -5608.4 -2443.9 -237.1 # of moles and mol percent K2O Al2O3 1 100% 0 0% 1 100% 0 0% 0 0% 0 0% 0 0% 1 100% 0 0% 0.5 100% 0 0% 0.5 100% 0 0% 1 33% 0 0% 1 20 % 0.5 25% 0.5 25% 0.5 17% 0.5 17% 0.5 12.5% 0.5 12.5% 0.5 10% 1.5 30% 0 0% 150 % Formula Compound SiO2 0 0% 0 0% 1 100% 0 0% 0 0% 0 0% 2 67% 4 80% 150 % 2 67% 3 75% 3 60% 150 % K-oxide K-hydroxide Quartz Corundum Gibbsite Boehmite Kaolinite Pyrophyllite Kaliophilite Leucite Microcline Muscovite Kyanite H2O K2O KOH SiO2 Al2O3 Al (OH)3 Al O (OH) Al2 Si2 O5 (OH)4 Al2 Si4 O10 (OH)2 KAlSiO4 KAlSi2O6 KAlSi3O8 KAl3Si3O10(OH)2 Al2SiO5 water
Plot the minerals on the diagram. Practice plotting a few until you are com- fortable with the pro- cedure. Next 2 slides re- view this procedure and the finished diagram is on the following slide. SiO2 Al2O3 K2O
Ca0.5Mg0.5CO3 50 mol% Ca 50 mol% Mg The chemistry is plotted where the two lines intersect. CaCO3 100% 0% MgCO3 0% FeCO3 MgCO3 100% FeCO3 100% 0% CaCO3
Ca0.2Mg0.4Fe0.4CO3 20mol%Ca 40mol%Mg 40mol%Fe CaCO3 100% 0% MgCO3 0% FeCO3 MgCO3 100% FeCO3 100% 0% CaCO3
Determine which mineral is stable at the 3 apices. (Use GR) {Any ideas how ?} Not considering amorphous silica minerals so you just go with quartz on top. What is stable at K apex? The reaction relating oxide and hydroxide is GO/NO-GO -just write the reaction between them. K2O to KOH K2O (s) + H2O (l ) KOH (s) Now calculate the Gr Gr = Gf (KOH) [ ( G f (K2O) ) + ( Gf (H20) )] = -379.1 [ (-322.1) + (-237.1)] = -99.5 kj/mol Goes as written - KOH stable - so it is at the lower left-hand corner. The order in which you write the reactions and calculate Gr does not matter It will work out correctly.
What is stable at the Al apex? Since the reactions relating 3 Al minerals are GO/NO-GO write the reactions between them. Al2 O3 (s)+ 1 H20 (l) GR = -8.3 AlOOH (s)+ H2O (l) GR = -2.2 The order in which you write the reactions and calculate GR does not matter It will work out correctly. Al (OH) 3 (s) Gibbsite stable Al (OH) 3 (s) Gibbsite stable
SiO2 quartz pyrophyllite microcline leucite kaolinite muscovite kyanite kaliophilite KOH Al(OH)3 gibbsite
Looks unmanageable, but when we eliminate un- stable collinear minerals it will simplify quite a bit. SiO2 quartz Now you must draw in all tie- lines connecting possibly stable coexisting phases. pyrophyllite microcline leucite kaolinite muscovite kyanite kaliophilite KOH Al(OH)3 gibbsite Examples of sets of collinear phases are indicated in lilac, gold, and green
SiO2 That is, is every segment between 2 minerals a tie- line? Triplets to be exam- ined by the method on the next slides are indicated in purple, green, and yellow. Deal with collinear phases on the outside edges of the diagram first. This will simplify extensively how many minerals you will have to deal with and will cut down the number of possible tie-lines. Determine which of the adjacent mineral pairs in each triplet is stable. quartz pyrophyllite kaolinite kaliophilite kyanite KOH Al(OH)3
Start at one apex and work down. Is pyrophyllite stable or does it breakdown to form kaolinite + quartz? Al2 Si2 O5 (OH)4 (s)+2SiO2 (s) GR = +9.7 Pyrophyllite is not stable. It breaks down to form kaolinite + quartz so it drops off the diagram. Is kyanite stable w.r.t. kaolinite + gibbsite? Al2 Si2 O5 (OH)4(s)+2Al(OH)3(s) GR = +36.6 Kyanite is not stable w.r.t. kaolinite + gibbsite so it drops off the diagram. Al2Si4O10(OH)2 (s)+ H2O (l) 2Al2 SiO5(s)+5 H2O(l)
Is kaolinite stable w.r.t. quartz + gibbsite ? 2SiO2 (s)+ 2Al(OH)3 (s) GR = -13.4 Kaolinite is stable w.r.t. quartz + gibbsite so it remains on the diagram When the central of the three minerals is stable it DOES NOT eliminate the two minerals on either side. Instead, all three are stable and remain on the diagram. Al2 Si2 O5 (OH)4 (s)+ H2O (l)
SiO2quartz Now that you have eliminated pyrophyllite and kyanite here are the remaining tie- lines connecting possibly stable coexisting phases. microcline leucite kaolinite muscovite kaliophilite KOH Al(OH)3 gibbsite
SiO2 quartz Now we have colored lines showing remaining triplets of collinear phases to test. kaolinite kaliophilite KOH Al(OH)3
Now start checking these internal collinear phases. leucite + quartz = microcline KAlSi2O6 (s)+ SiO2 (s) GR = -14.9 microcline is stable KAlSi3O8 (s) microcline + kaliophilite KAlSi3O8 (s)+ KAlSiO4 (s) GR = +5.4 leucite 2KAlSi2O6 (s) leucite is not stable kaliophilite + quartz KAlSiO4 (s)+ 2SiO 2 (s) GR =-24.4 microcline KAlSi3O8 (s) microcline is stable
microcline + gibbsite KAlSi3O8(s)+ 2Al(OH)3(s) GR = -29.5 muscovite KAl3Si3O10(OH)2(s)+ 2 H2O(l) muscovite is stable kaolinite + kaliophilite Al2Si2O5 (OH)4(s) + KAlSiO4(s) GR = -40.5 muscovite KAl3 Si3 O10 (OH)2(s)+H2O (l) muscovite is stable muscovite + KOH KAl3 Si3 O10 (OH)2 (s)+2KOH (s) GR = -123.5 kaliophilite 3KAlSiO4 (s)+2H2O (l) kaliophilite is stable
Now draw in all possible tie- lines connecting remaining minerals. These are crossing tie- lines and are not per- mitted by thermodyna- mics. We must write a SiO2 quartz microcline Note that the last two drawn (with dashed lines) intersect at a point where there is no mineral plotted. kaolinite chemical reaction to determine which tie-line remains on the diagram and which is eliminated. muscovite kaliophilite Al(OH)3 KOH Write the correct reaction before you look at the next slide.
SiO2 quartz KAl3 Si3 O10 (OH)2 (s)+2SiO 2(s)+H2O(l) KAl Si3O8 (s)+ Al2 Si2 O5 (OH)4 (s) Quartz + Muscovite is stable microcline muscovite + quartz microcline + kaolinite kaolinite muscovite kaliophilite GR = +16.1 KOH Al(OH)3
SiO2 quartz Here is the final diagram showing which minerals can be found coexisting stably in nature. microcline kaolinite muscovite kaliophilite gibbsite KOH Al(OH)3
SiO2 quartz KOH, kaliophilite, gibbsite Which compounds are stable at the polygons? muscovite, kaliophilite, gibbsite microcline kaolinite kaolinite, quartz muscovite kaolinite,gibbsite kaliophilite kaliophilite quartz gibbsite KOH Al(OH)3