Understanding Glycogenolysis in Vertebrates: A Detailed Overview
In vertebrates, glycogen serves as a glucose reservoir in the liver and skeletal muscles. Glycogenolysis is the breakdown of glycogen into glucose-6-phosphate, crucial for providing glucose to tissues in the absence of dietary sources. Enzymes like glycogen phosphorylase, debranching enzyme, and phosphoglucomutase play key roles in this process, converting glycogen to glucose for energy production.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Glycogenolysis Glycogenolysis Class name IVH Course name - ZOO-Biochemistry (Rohit)
Introduction Introduction In vertebrates, glycogen is found primarily in liver and skeletal muscles. Liver glycogen serves as a reservoir of glucose for other tissues when dietary glucose is not available. This is important for neurons of brain because these cells can not use fatty acid as source of fuel. Glycogenolysis is the process of breakdown of glycogen into glucose- 6-phosphate.
Glycogen breakdown requires following enzymes 1. Glycogen phosphorylase, 2. Glycogen debranching enzyme, 3. Phosphoglucomutase.
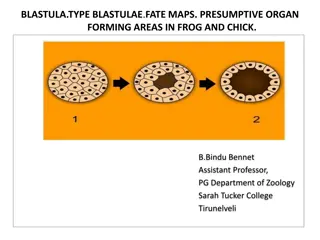
Glycogen Breakdown Is Catalyzed by Glycogen Phosphorylase Glycogen phosphorylase catalyzes the reaction in which an (1-4) glycosidic linkage between two glucose residues at a nonreducing end of glycogen undergoes attack by inorganic phosphate (Pi), removing the terminal glucose residue as D-glucose 1-phosphate. This is known as Phosphorolysis reaction (Because Inorganic phosphate (Pi) is used). This is different from Hydrolysis of glycosidic bonds by amylase as water molecules is involved in it instead of Pi. Pyridoxal phosphate is required as a cofactor in the glycogen phosphorylase reaction. Phosphate group of pyridoxal phosphate molecule promotes attack by Pi on the glycosidic bond. Glycogen phosphorylase acts repetitively on the nonreducing ends of glycogen branches until it reaches a point four glucose residues away from an (1-6) branch point, where its action stops.
Glycogen phosphorylase removes the terminal glucose residues from the non-reducing end of a glycogen chain.
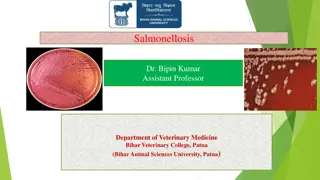
Debranching enzyme, formally known as oligo (1-6) to (1-4) glucantransferase, catalyzes two successive reactions that transfer branches. 1. First is the transferase activity, in which the enzyme removes terminal 3 of remaining glucose residues (Yellow) and transfer this to non-reducing end of another branch. 2. Second, the remaining glucose residue (Red) is removed glucosidase activity of debranching enzyme. by alpha(1-6) Once these branches are transferred and the glucosyl residue at C-6 is hydrolyzed, glycogen phosphorylase activity can continue.
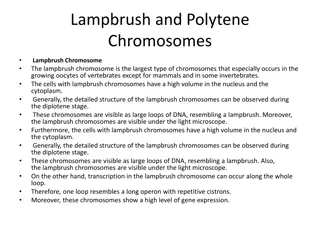
Glucose 1-phosphate (end product of the glycogen phosphorylase reaction) is converted to glucose 6-phosphate by phosphoglucomutase, which catalyzes the reversible reaction.
Regulation of glycogenolysis Two hormones are responsible for stimulating the breakdown of glycogen in muscle and liver. 1. Glucagon (secreted by pancreas) 2. Epinephrine (secreted by adrenal medulla of kidney) Liver is more responsive to glucagon whereas muscle is more to epinephrine. Glycogen phosphorylase is regulated Hormonally. glycogen phosphorylase of skeletal muscle exists in two interconvertible forms: 1. Glycogen phosphorylase a, which is catalytically active, and 2. Glycogen phosphorylase b, which is less active.
Regulation of Glycogen phosphorylase (Glycogenolysis) by Covalent modification (reversible phosphorylation which is responsive to hormones) During vigorous muscular activity the epinephrine phosphorylation specific Ser residue in phosphorylase converting it to its more active phosphorylase a. hormone triggers of When the muscle returns to rest, a second enzyme, phosphorylase a phosphatase, also called phosphoprotein phosphatase 1 (PP1), removes the phosphoryl groups from phosphorylase a, converting it to the less active form, phosphorylase b. a b, form,
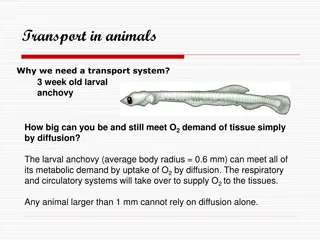
How hormones works in regulation of Glycogen breakdown 1. By binding to specific surface receptors, either epinephrine acting on a myocyte (left) or glucagon acting on a hepatocyte (right) activates a GTP- binding protein Gs It triggers cAMP In response to cAMP, protein kinase A get activate Finally phosphorylase b kinase becomes active and in turn activate Glycogen phosphorylate a (Active form) Glycogen breakdown initiated
Glucogen synthase (Glycogenesis) regulation by phosphorylation/dephosphorylation Like glycogen phosphorylase, glycogen synthase can exist in phosphorylated and dephosphorylated forms Its active form, glycogen synthase a, is unphosphorylated. Phosphorylation of the hydroxyl side chains of several Ser residues of both subunits converts glycogen synthase a to glycogen synthase b, which is inactive. Glycogen synthase kinase 3 (GSK3) is responsible for phosphorylation of serine residue of Glycogen synthase and making it inactive.
Glycogen synthase kinase 3 works only when another kinase called casein kinase II (CKII) phosphorylate Glycogen synthase on nearby residues and this process is known as priming. Phosphoprotein phosphatase 1 (PP1), converts glycogen synthase b to the active form. Glucose 6-phosphate binds to an allosteric site on glycogen synthase b, making the enzyme a better dephosphorylation by PP1 and causing its activation. substrate for
Phosphoprotein Phosphatase 1 Is Central to Glycogen Metabolism A single enzyme, PP1, can remove phosphoryl groups from all three of the enzymes phosphorylated in response to glucagon (liver) and epinephrine (liver and muscle): phosphorylase kinase, glycogen phosphorylase, and glycogen synthase. PP1 is itself subject to covalent and allosteric regulation. It is inactivated when phosphorylated by PKA and is allosterically activated by glucose 6-phosphate