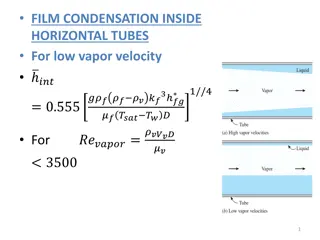
Understanding Heat Transfer in Phase Changes of Water
Water molecules exhibit different behaviors in the liquid and gaseous states due to varying attractions between molecules. To change liquid water to a gas, energy must be added to overcome intermolecular forces, making this process endothermic. The heat absorbed during melting is equal to the heat released during solidification, illustrating the principle of conservation of energy. Additionally, the molar heat of fusion and solidification play crucial roles in phase transitions in chemistry. Furthermore, the molar heat of vaporization and condensation highlight the energy requirements for converting substances between gas and liquid states.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
What is the difference between water molecules in the liquid state compared to the gaseous state? Are liquid water molecules attracted to one another? What must happen, then, for liquid water to change to a gas? So, is this state change endothermic or exothermic?
What is the difference between water molecules in the liquid state compared to the gaseous state? In gas state the molecules are farther apart and not as strongly attracted to each other. Are liquid water molecules attracted to one another? YES What must happen, then, for liquid water to change to a gas? Energy must be ADDED to overcome the attraction So, is this state change endothermic or exothermic? ENDOTHERMIC
All solids absorb heat as they melt to become liquids (ex. ice to water) This results in a change of state rather than change of temperature The temperature remains constant Molar heat of fusion is the amount of heat required to melt one mole of substance Hfus Molar heat of solidification is the amount of heat released to convert a liquid to a solid. Hsolid
The quantity of heat absorbed by a melting solid is the exact same as the amount of heat released when a liquid solidifies Figure 17.9, page 520
Which arrows represent processes that release heat to the surroundings?
H2O(s) H2O(l) Hfus = 6.01kJ/mol H2O(l) H2O(s) Hsolid = -6.01kJ/mol
The amount of heat required to vaporize one mole of substance is called the molar heat of vaporization Hvap This value is typically much higher that the molar heat of fusion The amount of heat released by one mole of substance as it cools from a gas to a liquid is called its molar heat of condensation Hcond
The quantity of heat absorbed by a vaporizing liquid is the exact same as the quantity of heat released when the vapor condenses to a liquid
H2O(l) H2O(g) Hvap = 40.71kJ/mol H2O(g) H2O(l) Hcond = -40.71kJ/mol
During the formation of a solution (aq), heat is either released or absorbed The enthalpy associated with the dissolution of one mole of substance is the molar heat of solution Hsoln A dissociation equation can be written to illustrate this action
Hot pack - exothermic In which direction is the heat flowing? Cold pack - endothermic In which direction is the heat flowing?
Questions 21 22, page 521 Answer the questions for interpreting graphs page 523 Fig 17.10 Questions 23 24, page 524 Guided reading, section 17.3 A. How much heat must be removed to freeze a tray of ice cubes if the water has a mass of 50.0g? B. How many kilojoules of heat are required to vaporize 50.0g of ethanol (C2H5OH)?
https://ed.ted.com/lessons/how-do-cold-packs-get-cold-so-fast-john-pollardhttps://ed.ted.com/lessons/how-do-cold-packs-get-cold-so-fast-john-pollard https://ed.ted.com/lessons/how-do-cold- packs-get-cold-so-fast-john-pollard
Page 523 Examine the graph and answer the questions on the right side of the figure
Answer the interpreting graphs questions How would an increase in the rate of heating affect the heating curve?
A) Constant temperatures at the melting point and the boiling point B) It takes much less energy to melt a given mass of ice than to vaporize the same mass or water. C) The plateaus on the graph represent coexistence of solid and liquid and liquid and vapour The rate of heating the faster the rate of heating the steeper the slope will be.
Complete questions 25- 31 from page 526 Complete problem sheet Heat in Changes of State 5 problems
https://www.ck12.org/chemistry/Multi-Step-Problems-with-Changes-of-https://www.ck12.org/chemistry/Multi-Step-Problems-with-Changes-of- State/lesson/Multi-Step-Problems-with-Changes-of-State-CHEM/