Understanding Reaction Rates in Chemistry: Apparatus, Experiment, and Analysis
Explore the concept of reaction rates in chemistry through the use of a gas syringe apparatus, conducting experiments, analyzing results, and understanding factors affecting reaction rates. Dive into hands-on activities and graphical representations to enhance your understanding of this fundamental chemical concept.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
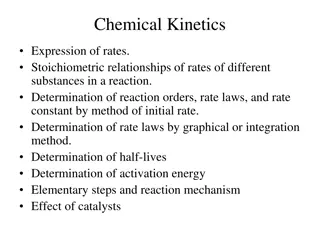
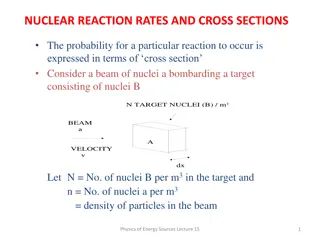
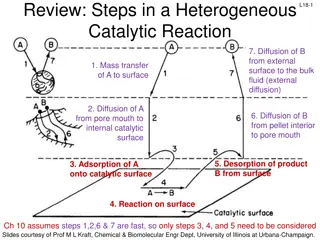
Reaction Rates WJEC Chemistry Unit 1 - 1.5 WJEC Science (Double Award) Unit 2 - 2.5
The apparatus: gas syringe Try and explain to a fellow classmate what you think this apparatus does. Click the button to start the experiment and see what happens. Start experiment Were you right? acid gas granules What other apparatus do you need in order to investigate what factors affect the reaction rates? conical flask
Trial run: Click the button to start. Start Can you explain what the graph is showing us? stop clock What are the main features of the line? volume of gas/cm graph of results What changes could you make that would affect the result? 10 8 6 4 2 0 time/s 90 180 150 0 30 60 120
Here are a set of actual results. Plot them on the graph below using the pen tool: Click for help on filling the axes Click for help on filling the axes Time (s) 0 1 2 3 4 5 6 7 8 9 10 Volume of gas (cm3) 0 13 26 35 44 51 56 61 65 68 70
Here are a set of actual results. Plot them on the graph below using the pen tool: Time (s) 0 1 2 3 4 5 6 7 8 9 10 Show x axis Volume of gas (cm3) 0 13 26 35 44 51 56 61 65 68 70 Show y axis 70 Now draw the points or 60 Volume of gas (cm3) 50 Click to see the plot points 40 30 Now draw the curve 20 Click to check answer 10 Time (s) 10 20 30 40 50 60 70 80 90 100
Reaction Rates Click the red buttons to choose the factor Start Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs
Reaction Rates Click the red buttons to choose the factor Start Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs
Reaction Rates Click the red buttons to choose the factor Start Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs
Reaction Rates Click the red buttons to choose the factor Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs What can you deduce from these graphs? Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs
Reaction Rates Click the red buttons to choose the factor Start Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs
Reaction Rates Click the red buttons to choose the factor Start Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs
Reaction Rates Click the red buttons to choose the factor Start Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs
Reaction Rates Click the red buttons to choose the factor Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs What can you deduce from these graphs? Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs
Reaction Rates Click the red buttons to choose the factor Start Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs
Reaction Rates Click the red buttons to choose the factor Start Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs
Reaction Rates Click the red buttons to choose the factor Concentration of acid 0.5 mol. dm 1.0 mol. dm 1.5 mol. dm All three graphs What can you deduce from these graphs? Temperature 20 C 25 C 35 C All three graphs volume of gas/cm 10 8 6 4 2 0 Surface Area Granules time/s 90 180 150 0 30 60 120 Powder Both graphs