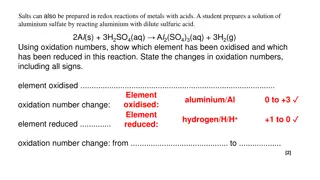
Chemical Reactions: Vocabulary Practice and Experiments in Chemistry 101
In this additional text for practicing chemical reactions vocabulary, students in Chemistry 101 conduct an experiment by combining a solvent and a solute to observe a simple chemical reaction, focusing on the proper process and product description. The materials, instructions, and objectives are provided, emphasizing safety measures and avoiding excess reactants. Students read instructions, mark statements as true or false, and match key vocabulary terms with definitions to enhance comprehension.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Chemical Reactions Chemistry 101 This is an additional text plus exercises for practicing vocabulary related to chemical reactions
Chemistry 101 Each student receives the same materials for this assignment. You will combine a solvent and a solute. If you follow the instructions, the solution creates a simple chemical reaction. Add the limiting reactant slowly to avoid a safety hazard. NOTE: This lab will NOT require additional catalysts. For next week s lab, we ll experiment with adding heat.
Chemistry 101 Objectives: - Learn the proper process to create a chemical reaction. - Be able to describe the product in your own words.
Chemistry 101 Materials: - 400ml of water, your reactant - 3g of sulfuric acid, your reagent - 2 pre-filled beakers
Chemistry 101 Instructions: - Add 1g of sulfuric acid to 300ml of water. Adding sulfuric acid in excess of 1g at this point is not recommended. - Add 100ml of water. The concentration of sulfuric acid to water should be low. - Add the remaining 2g of sulfuric acid. - Observe the reaction and report its yield.
Reading Read the instructions. Then, mark the following statements as true (T) or false (F). 1. Students will utilize burners in this experiment. 2. Students are instructed to add sulfuric acid to water. 3. Each student s materials should create a different reaction.
Key words: Solvent rastvarac Solute rastvorak Solution rastvor Reaction reakcija Reactant reaktant Reagent reagens In excess visak Yield prinos
Vocabulary Match the words or phrases (1-6) with the definitions (A-F) 1 yield 2 reaction 3 reactant Awhen two or more substances combine in a chemical event B the amount of something produced during a process C the number of molecules of a substance in a given volume D a substance that changes during chemical activity E a substance that is completely consumed and stops a reaction F a substance that dissolves into a solvent 4 solute 5 concentration 6 limiting reactant