Preparation of Acetanilide: A Detailed Overview
Acetanilide is synthesized through the acetylation of aniline using acetic anhydride and anhydrous sodium acetate, resulting in the formation of glistening plates upon cooling. Acetylation is preferred over other methods to control reactivity, and the mechanism involves the role of HCl. By deactivating the aromatic ring, mono-substitution at the para position is achieved. Properties of acetanilide and the importance of aniline's free form are also discussed in the process.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
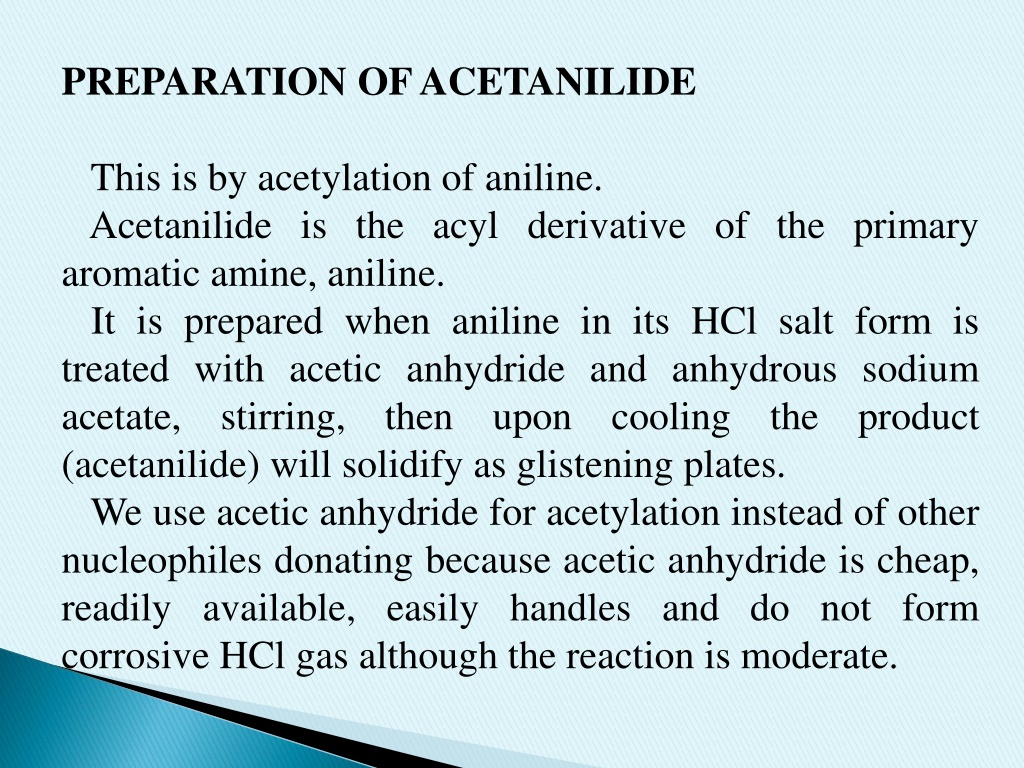
PREPARATION OF ACETANILIDE This is by acetylation of aniline. Acetanilide is the acyl derivative of the primary aromatic amine, aniline. It is prepared when aniline in its HCl salt form is treated with acetic anhydride and anhydrous sodium acetate, stirring, then upon cooling the product (acetanilide) will solidify as glistening plates. We use acetic anhydride for acetylation instead of other nucleophiles donating because acetic anhydride is cheap, readily available, easily handles and do not form corrosive HCl gas although the reaction is moderate.
Acetylation is done before chlorosulfonation because aniline can t be monosubstituted at p-position only because it is very reactive, e.g. aniline nitration give trinitroaniline, so we can t get monosubstituted sulfonamide. In aniline Electrophilic substitution occurs on o- and p-position but in Acetanilide , In part of time, electrons are shared with the ring and sometimes, they are shared with C of carbonyl which is cause electron deficient due to polarization.
Also, in order to decrease activity of aromatic ring (deactivation of the aromatic ring) by decreasing electronic density on the ring particularly on the 2- ortho position. This is done by acetylation of aniline where the acetyl group will exert inductive effect and steric hindrance effect, then monosubstitution on the p- position will be obtained. O O O O O HN C CH3 HN C CH3 HN C CH3 HN C CH3 HN C CH3
Mechanism of the reaction (role of HCl): HCl acid is added to solubilize aniline at the beginning since aniline is water-insoluble, so we add HCl to get anilinium chloride which is water-soluble. HCl is used to activate acetic anhydride since there is no reaction between aniline and acetic anhydride. NH2 O O + H3C C O C CH3 N.R.
In this case we need aniline in the free form (i.e., the 2 electrons of N are free), and in order to obtain free aniline to attach to acetic anhydride to give active acetanilide, we must liberate it by the addition of weak acid salt (anhydrous sodium acetate) which liberates aniline, then the 2 electrons of N are free to attach. NH2 NH3Cl + CH3COO-Na+ + HCl + CH3COO- + NaCl .. H O O H N C O C CH3 NH2 O O CH3 + H3C C O C CH3 O : NH C CH3 + CH3COOH Acetanilide Acetic acid
Properties of acetanilide: 1. It crystallizes in colorless glistening plates. 2. It is sparingly soluble in water, easily soluble in alcohol to give neutral solution. 3. Hot dilute acids or alkalis slowly hydrolyze acetanilide to aniline and acetic acid or their salts. NH2 O dil. NaOH + CH3COONa NH C CH3 NH3Cl dil. HCl Acetanilide + CH3COOH
4. Melting point is 114 C. 5. gives yellowish to white precipitate of p-bromine acetanilide solution when react with bromin . This test is given to distinguish between acetanilide and phenacetin and paracetamol.
O O NH C CH3 NH C CH3 + Br2 Br p-Bromoacetanilide Acetanilide O NH C CH3 + Br2 N.R. O C2H5 Phenacetin O NH C CH3 + Br2 N.R. OH Acetaminophen
Procedure: 1. Add 4.9 ml aniline to 135 ml H2O + 4.5 ml conc. HCl. 2. In another small beaker dissolve 0.5 g of anh. sod. acetate with 30 ml H2O, add this to the aniline mixture. 3. Add 6.5 ml acetic anhydride gradually. 4. Mix and cool for 5 min. until white glistening plates of acetanilide appear. 5. Filtrate. 6. If we want recrystallization of acetanilide use hot water or mixture of water/alcohol. 7. Calculate % of yield.