Exploring Solids and Properties of Matter
Dive into the world of solids and properties of matter, from seating arrangements to classroom objectives. Discover the different phases of matter, investigate properties of solids, liquids, and gases, and explore the interactions between particles. Engage in hands-on activities and learning experiences to deepen your understanding of the fascinating world of chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Do Now: Find new seats melanie 1 zacharia 9 jacob 17 angelica 25 andretta 2 ian 10 arianna 18 jennifer 3 joy 11 gabrielle 19 daniel 26 elias 4 Shajra 12 Jose 20 mariah 5 jairo 27 Nailah 21 de'andre 13 casandra 6 syndey 22 hector 14 christina 28 shaquom 7 emily 23 julianna 15 augustin 8 Se'jean 24 kayla 29 isabella 16
Do Now: Find New Seats egar 1 jordan elijah yasheema joshua mina christopher kevin bruno 9 10 11 12 13 14 15 16 joseph seth johanan lindsey itzel Jessica jonathan vernon 17 18 19 20 21 22 23 24 parker 25 alexandra 2 damien 3 megan 26 kiara 4 lorena 5 sara 27 denzell 6 nazjai 28 olivia 7 emma 8
WELCOME BACK! WELCOME BACK! Bellringer: Read the first page of the notes packet and fill in the particle diagrams based on the reading Remember about gravity
Homework: Make sure you finish and turn in types of reactions lab by tomorrow
This unit All about phases, their properties and phase changes Are there different types of solids? What happens when things go from s l g? How much energy is needed in order to go through those changes
Todays objective Describe the properties of solids Design a lab to determine the type of solid for an unknown
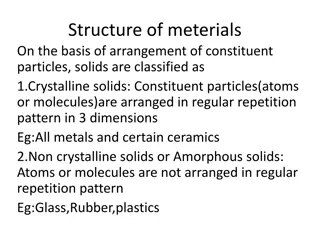
Solids High, Medium, or Low attraction between each particle? How do they move?
Liquids High Medium or Low attraction How do they move? Proof that liquid molecules are attracted to each other: http://www.youtube.com/wa tch?v=r7fEHYkGxd0
Gas High Medium or Low attraction? How do they move?
Solids! We are going to focus on Different types of solids. Focus: You are going to design a lab to determine what type of solid two different solids are Read along with your neighbor and fill in the venn diagram on the next page Maybe split it: 2 types per neighbor? Things all Solids have in common goes in the center Overlap is for things in common
Do Now Pick up a copy of the lab RETURN ANY EXTRA COPIES OF THE NOTES THAT YOU MAY HAVE TAKEN Make sure the following are described in the venn diagram: Melting point (very high, high, variable, low) Solubility Conductivity (if so, under what conditions) How the particles are arranged
Types of solids lab 2 different solids You have: Distilled water (may have CO2Dissolved in it ) This would make it slightly conductive need to account for that Conductivity testers cups stirring rods other miscellaneous lab equip (NOT BUNSEN BURNERS)
Procedure Get it approved before beginning It would be unfortunate to get all the way done and then find an error with the procedure and need to do it all over Something to think about: When CO2dissolves into water, it makes the water conductive going to need to account for that
Do now Take out your Types of Solids Lab Refresh your memory Ionic Molecular Sol. Cond. M.p. Network Metallic Make sure you get your procedure approved and finish the lab (and turn it in)
Absent? Then you need to play a little catch up Read the notes about the types of solids and fill in the 4 box ven diagram on the next page Use this information to figure out how you could test these 2 unknown solids to determine what type of solid they are
Homework: Finish the lab
Do now Bellringer Quiz: Pick up, do independently and turn in Also turn in Types of Solids lab
Homework Phases and phase change diagrams Heat of phase changes (front and back of 1stpage)
And now Read page 4 and fill in the graphic organizer on page 5 and 6
A nice simulator http://phet.colorado.edu/en/simulation/state s-of-matter-basics
Phase Changes: Label the phase changes (1-6), identify each side as either exothermic or endothermic, fill in the phase change diagrams and particle diagrams for each phase. _____thermic + or - H _____thermic + or - H Temp. (K) Temp. (K) Gas (particle diagram) Time Time 5.___________ 1.___________ 6.___________ 2.___________ Liquid (particle diagram) 3.___________ 4.___________ Solid (particle diagram)
Sublimation and Deposition http://www.youtube.com/watch?v=4E0sy- FN2M8
1) Label the phases present at each line segment above using (s), (l), and (g). 2) What is the boiling point of this substance? ________ 3) What is the melting point of this substance? ________
c. Heat of phase change In previous units we have calculated the amount of energy needed to change the temperature of water using the formula: q=mC T (remember that catchy song?). During a phase change, however, there is no change in temperature so this formula cannot be used to solve for the heat needed for a phase change. Instead, there are different formulas used for phase changes; q=mHf and q=mHv. Hf is the heat of fusion, the amount of energy required to melt or freeze a gram of water (334 J/g). Hv is the heat of vaporization of water; the amount of energy required to boil or condense water (2260 J/g).. m is the mass of water involved in the phase change.
How much energy is required to melt 50.0g of water at 0oC? q = m Hf Why Hf? q = 50.0 g x 334J/g q =16700 J
Practice Problems _____1) Which of the following phase changes requires heat of fusion to accomplish? a) H2O (s) H2O (g) c) H2O (l) H2O (g) _____2) Which of the following phase changes is endothermic? a) H2O (s) H2O (l) c) H2O (l) H2O (s) d) H2O (g) H2O (s) b) H2O (g) H2O (l) d) H2O (s) H2O (l) b) H2O (g) H2O (l)
Calculate the number of joules required to (show correct numerical setup): a) melt 20.0 g of H2O (s) at 0oC b) boil 30.0 g of H2O (l) at 100oC c) freeze 200.0 g of H2O (l) at 0oC d) boil 50.0 g of H2O (g) at 100oC
Ice cubes at 12.0oC are placed in a saucepan and heated at a constant rate over a stove to 115.0oC. Sketch a phase change diagram for the phase changes that occur between -12.0 oC and 115.0oC. Label the temperatures at which the phase changes occur. Then label each line segment with a letter (A, B, C, D, E, etc.).
Label where P.E. increases/stays same Do the same for K.E.
Do Now: Pick up a copy of the lab Read over the procedure to the lab
Questions? _____1) Which of the following phase changes requires heat of fusion to accomplish? a) H2O (s) H2O (g) b) H2O (g) H2O (l) (s) H2O (l) c) H2O (l) H2O (g) d) H2O _____2) Which of the following phase changes is endothermic? a) H2O (s) H2O (l) b) H2O (g) H2O (l) d) H2O (g) H2O (s) c) H2O (l) H2O (s) Calculate the number of joules required to (show correct numerical setup): a) melt 20.0 g of H2O (s) at 0oC boil 30.0 g of H2O (l) at 100oC b) freeze 200.0 g of H2O (l) at 0oC c) boil 50.0 g of H2O (g) at 100oC d)
Ice cubes at 12.0oC are placed in a saucepan and heated at a constant rate over a stove to 115.0oC. Sketch a phase change diagram for the phase changes that occur between -12.0 oC and 115.0oC. Label the temperatures at which the phase changes occur. Then label each line segment with a letter (A, B, C, D, E, etc.).
Safety Goggles Be careful of HOT WATER Don t drop the thermometers Do not poke a whole in the bottom of the calorimeters! Don t eat the ice
Big Idea Starting with hot water and Ice Going to calculate how much energy was lost by the water (q=mC T) That energy was used to melt the ice Calculate the Hf based on that q/(g of ice melted) Follow the procedure!
Do Now Take out The Heat of Fusion Lab from yesterday and begin working on it Plan on the Test being on Thursday Quiz on THIS FRIDAY (covering phase changes)
This periods agenda Finish the lab Finish the H.W. , front and back of the first page Start reading the 4. Gases and Pressure
Do now Turn in Heat of Fusion Lab Turn in first page (front and back) from homework packet Read over topic 4: Gases and Pressure (a+b) Start brainstorming on how you could visualize each of these concepts
Kinetic molecular theory (KMT) for gases and ideal gas laws Before we can discuss what an Ideal gas is, we must first discuss what is true for all gases, ideal and real. The following is true for all gases: Gas particles have no definite volume Gas particles have no definite shape Gases are highly compressible Gases take the volume and shape of the container Gas molecules are relatively far apart from one another Gases form homogeneous mixtures with each other
Real gases will act like Ideal gases at low pressure and high temperature. Smaller gases (H2 and He) also behave more ideally than larger gases (CO2, CH4).
The concept of the Ideal gas is to explain the behavior of real gases. There is a list of things that we assume about gases to be true to explain their behavior. For example, we assume that ideal gas particles have no attraction for each other, which is why the take on the volume of their container. Real gas particles do have some attraction to each other. The reason we make these assumptions is because they are mostly true, and in doing so we can calculate a lot of information about gases. The following are the Ideal Gas Laws:
Gas molecules are so small that the combined size is insignificant compared to the volume occupied by the gas. Gas molecules move in straight line motion until they collide with the container wall or another gas molecule Any collisions between gas molecules are elastic with NO energy lost from the collisions. No attractive or repulsive forces exist between gas molecules. The Average velocity (speed with direction) of the gas is directly proportional to the KELVIN temperature (the higher the temperature, the faster they move).
b. Avagadros hypothesis Simply put, Avogadro s Hypothesis states that equal volumes of ALL gases, at the same temperature and pressure, have the same number of molecules. If you were to double the number of molecules, you would double the volume.
Poster Project (14 minutes) Illustrations are excellent for visualizing some abstract concepts. You will use your creativity to create a visual for one of these concepts It can be a literal representation, an example from real life or a metaphor. Example: The atom is mostly empty space Draw an atom with mostly empty space A club named Atom , which is almost completely empty
Poster Project (14 minutes) Be prepared to explain your illustrations to the class
Quickly Sketch your favorites into your notes packet
Do Now (Test Thursday) Take out Homework Packet and put everything else away Homework check time! Finished? Read: Pressure, vapor pressure and boiling points through Using the reference tables Try some of the practice