Understanding Ligand Substitution Reactions in Metal Complexes
Ligand substitution reactions in metal complexes involve the replacement of one ligand with another, impacting the coordination around the metal center. The mechanism of these reactions, associative or dissociative, is influenced by factors like coordination saturation and electron count. Experimental data guides the proposed mechanisms, which are subject to validation. Key pathways like ligand addition and dissociation play crucial roles in these dynamic processes, shaping the behavior of metal complexes during ligand exchanges.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
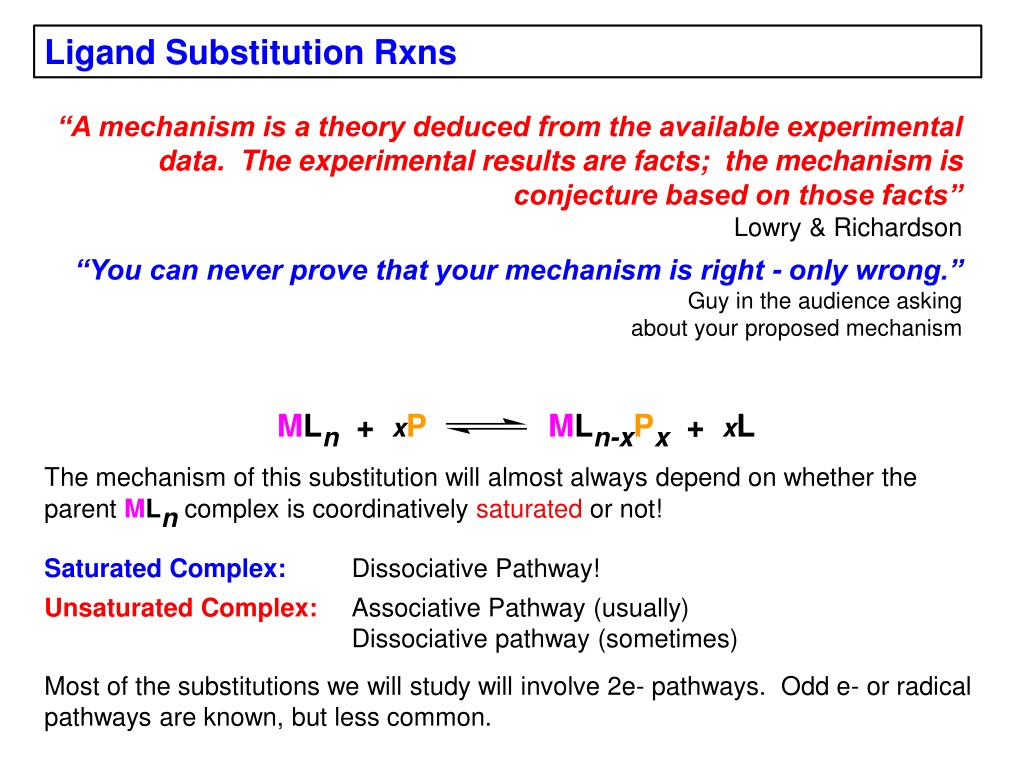
Ligand Substitution Rxns A mechanism is a theory deduced from the available experimental data. The experimental results are facts; the mechanism is conjecture based on those facts Lowry & Richardson You can never prove that your mechanism is right - only wrong. Guy in the audience asking about your proposed mechanism MLn + xP MLn-xPx + xL The mechanism of this substitution will almost always depend on whether the parent MLn complex is coordinatively saturated or not! Saturated Complex: Unsaturated Complex: Dissociative Pathway! Associative Pathway (usually) Dissociative pathway (sometimes) Most of the substitutions we will study will involve 2e- pathways. Odd e- or radical pathways are known, but less common.
Ligand Addition (association): this is when an incoming ligand coordinates to a metal center that has one or more empty orbitals available. Ligand Addition Ph3P Cl PPh3 Ph3P Rh + CO Rh Cl OC Ph3P This Rh(+1) complex is d8 and only 14e-. Adding a ligand takes one to the more stable 16e- square-planar complex. Ligand Dissociation Ph3P Ph3P Cl PPh3 Ligand Dissociation: this is when a ligand coordinated to a metal dissociates (falls off). The probability of a specific ligand dissociating depends on how strongly or weakly it is coordinated to the metal center and steric effects. Rh + PPh3 Rh Cl Ph3P Ph3P The steric hindrence of the three bulky PPh3 ligands favors dissociation of one to form the 14e- RhCl(PPh3)2 complex. The moderate electron-donating ability of the PPh3 ligand (not a strongly coordinating ligand) makes this fairly facile. Cl Cl Me2 P Me2 P Me2 P Me2 P + Cl Ru Ru P Me2 P Me2 P Me2 P Me2 Cl The strongly donating ability of the dmpe ligands combined with their strong chelate effect makes it difficult to dissociate one of the PMe2 arms. In this case the Cl- anion is the one that dissociates, leaving a cationic complex behind. The two dmpe ligands donate enough electron-density to the Ru center to make it reasonable to dissociate a Cl-.
A ligand substitution can occur either by an associative or dissociative route. The exact mechanism depends in large part on the electron-count of the metal complex undergoing the ligand substitution. The simplest case is when one is dealing with an 18e- metal complex. In this case one almost always has a dissociative substitution. O O O C C C -CO +PMe3 OC CO OC CO OC CO Mo Mo Mo OC CO OC CO OC CO C O PMe3 18e- saturated complex 16e- unsaturated complex 18e- saturated complex incoming ligand O O L L C C Almost NO evidence for this type of rxn: OC CO OC CO OC CO Mo + CO Mo + L Mo OC CO OC CO OC CO C O C O C O about to be dissociated ligand 18e- complex 20e- transition state with added ligand 18e- complex
Dissociative substitution can also occur in 16e- (or in very unusual cases, lower electron count systems) complexes. These cases either involve sterically bulky ligands that block the open coordination site, or third row square planar d8 complexes like Pt(+2) where there are strong electronic factors that limit the coordination of an additional ligand to the empty axial site. -PCy3 +PMe3 Cl Cl PCy3 Cl Cl PMe3 Cl Ni Ni Ni Cy3P Cl Cy3P Cy3P 14e- unsaturated complex 16e- unsaturated complex 16e- unsaturated complex The large PCy3 ligands sterically block access to the empty axial pz orbital
+Br -Cl Cl PPh3 Cl Cl PPh3 Cl PPh3 Br Pt Pt Pt Ph3P Ph3P Ph3P +Br Br -Cl Cl PPh3 Cl Cl PPh3 Br Pt Pt Ph3P Ph3P The spatially extended filled axial Pt dz2 orbital partially blocks coordination of ligands via the empty axial pzorbital. This limits ligand association, although it can occur. Problem:The rate of substitution reactions on square planar d8 complexes goes in the order: Ni > Pd >> Pt. Explain why.
Steric Factors KD Ni(PR3)4Ni(PR3)3 + PR3 25 C Ligand: P(O-p-tolyl)3 128 P(O-i-Pr)3 130 P(O-o-tolyl)3 141 P(OEt)3 109 PPh3 145 Cone angle: < 10 10 6 x 10 10 2.7 x 10 5 4 x 10 2 > 1000 KD: Problem:There is also an electronic effect in the this example that favors PPh3 dissociation. What is it?
Solvent Effects -Cl + solvent Cl PPh3 Cl PPh3 Cl Cl PPh3 Pt Pt Pt Ph3P Ph3P Ph3P solvent - solvent +Br Cl PPh3 Br Pt Ph3P
Trans Effect The trans effect concerns the electronic effect of one ligand on another ligand when they are trans (opposite) to one another. The classical trans effect involves two -donating ligands trans to one another. -Cl N N Cl PEt3 L PEt3 L PEt3 L Pt Pt Pt Et3P Et3P Et3P Relative rate of substitution based on trans ligand L : Cl = 1, Ph = 100, CH3 = 103, H = 104 There is a cis effect, but it is much weaker and basically ignored: -Cl N Et3P Et3P Cl L N Et3P Pt Pt Pt Et3P Et3P L Et3P L Relative rate of substitution based on cis ligand L : Cl = 1, Ph = 2, CH3 = 4, H = 4
Note that when most chemists talk about the trans effect they are referring to the - type of trans effect, where a strong -donor weakens the -donating ligand trans to it. Do NOT overestimate the importance of the trans-effect. There are other forms that have different effects. -Acceptor Trans Effects Trans effects that involve -backbonding ligands. CO ligands represent the most common type. -backbonding to a metal is strengthened when it is trans to a good -donating ligand that can t - backbond -backbonding to a metal is weakened when it is trans to another good -backbonding ligand
-Pushing Effect There is a further strengthening of M-CO -backbonding when the trans ligand has -donation properties that can push up the energy of the filled d orbitals and, in turn, make them better -donors to the CO. This can occur even when the ligand is not an especially strong donor. An example of this can be seen in the following three complexes and their anomalous CO stretching frequencies: PPh2 PPh2 S O PPh2 Ph2P Ph2P Ph2P Rh Rh Rh C I C I C I O O O CO = 2011 cm-1 CO = 1987 cm-1 CO = 1983 cm-1
Problem: Consider the following series of substitution reactions. O O PMe3 PMe3 C C PMe3 PMe3 PMe3 O O O O O O O C C C C C PMe3 C C Cr Cr Cr Cr PMe3 PMe3 C PMe3 80 C C C C C 25 C 110 C O O O O O C O C O C O C O As one replaces each CO ligand with a PMe3, the next CO substitution is progressively more and more difficult requiring higher temperatures and longer times. Once one forms Cr(CO)3(PMe3)3, it is extremely difficult to replace another carbonyl ligand. Why? Give all the major reasons?
Associative Substitutions These occur first by a ligand addition to the metal complex followed by the dissociation of one of the original ligands. You typically need to have an unsaturated (17e- or lower) complex in order to propose an associative substitution mechanism. PMe3 -PPh3 +PMe3 OC OC OC PMe3 Cl PMePh2 Cl PMePh2 Cl Rh Rh Rh Ph2MeP Ph2MeP Ph2MeP 18e- saturated complex 16e- unsaturated complex 16e- unsaturated complex -Cl OC PMePh2 PMe3 This rxn could proceed by two different likely substitution routes. Cl- is not that strong a ligand and can be substituted off by stronger donating neutral ligands like PMe3. Rh Ph2MeP 16e- unsaturated complex The filled axial Pt dz2 orbital partially blocks coordination of ligands via the empty axial pz orbital. This limits, but does not stop ligand association, which is quite common for Rh(I) and Pd(II).
AC/DC Ligands Multidentate ligands (those donate more than 2e- and occupy more than one coordination site on a metal) can often change their coordination number to donate fewer electrons, thus opening up a coordination site that can allow an associative substitution (or just ligand addition). 1-Cp H Casey & coworkers Keq = 0.4 H3C PMe3 + 2PMe3 Re Re ON Me3P PMe3 CH3 4 C Me3P N O PMe3 50 C 80 C PMe3 PMe3 CH3 THF Me3P PMe3 Re Re PMe3 ON 3-Cp Me3P PMe3 0-Cp N O CH3 Me3P
Indenyl Effect The indenyl ligand family, however, shows dramatically enhanced substitution reactions due to the ability to switch the aromaticity between the Cp and arene ring via the following resonance structures: indenyl resonance/aromaticity switch
The indenyl effect dramatically lowers the barrier for the 5-Cp to 3-Cp resonance structure, opening up a free coordination site and allowing far easier ligand additions and substitution reactions. + PPh3 Rh Rh Rh C CO C CO PPh3 C O O O C O - CO Rh Rh C C O PPh3 PPh3 O
Pentadienyl The pentadienyl ligand is an acyclic version of Cp that does not have any aromatic stabilization. This has two important effects: 1) No aromatic stabilization means that the -orbitals are higher in energy and are, therefore, better donors than Cp . Similarly, the *-antibonding orbitals are lower in energy and are better -acceptors than Cp (but the low electronegativity limits the amount of -backbonding that can occur). 2) The lack of aromatic stabilization means that there is a much smaller barrier for 5-pentadienyl 3-pentadienyl 1-pentadienyl transformations. Bleeke & coworkers PMe3 PMe3 Me3P PMe3 Me3P Re Re PMe3 Re Me3P PMe3 Me3P PMe3 PMe3 PMe3 Me3P Me3P 5 3 1
Nitrosyl We usually count the nitrosyl ligand as a cationic 2e- donor, isoelectronic with CO. But it can adopt an anionic 2e- configuration with a bent coordination geometry: metal has n electrons metal has n-2 electrons M N M N O O The nitrosyl ligand can shift from linear to bent, cationic to anionic, and open up a coordination site on the metal by essentially oxidizing it (shuttling 2e- from the metal to the NO+ turning it into NO ). The linear NO+ form can usually be easily differentiated from the bent anionic form by IR spectroscopy because of the large change in NO bond order (triple to double bond). O O O O N + L N N N N Fe Fe O Fe L O N CC O C C O CC O O O O 18e- (Fe2 ) 18e- (Feo) 16e- (Feo)
Radical Odd Electron Systems 17e- systems: One typically sees fairly dramatic rate enhancements for ligand substitution reactions of 103 to 107 compared to 18e- systems. molten PPh3 18e- [V(CO)6] + PPh3 no reaction ! 150 C [V(CO)5(PPh3)] + CO [V(CO)6] + PPh3 17e- -70 C The mechanism for the 17e- [V(CO)6] radical is believed to be associative to give a 19e- complex. The 19e- configuration weakens and labilizes one of the V-CO bonds allowing a CO to dissociate, dropping the complex back to a 17e- configuration. This is supported by the following experimental data: Rate = k[V(CO)6] [PPh3] (second order) S = 28 J/mol K (negative entropy indicates ordered transition state)
Electron Transfer Catalysis (ETC) - 1e + PPh3 Mn Mn Mn N PPh3 C C N N C O O Me C O C O C O C C C O Me Me 18e- 17e- 19e- - NCMe + 1e Mn Mn PPh3 PPh3 C C O O C O C O 17e- 18e-
Problem: One could use electron transfer catalysis (ETC) to further activate the very inert trans-Cr(CO)3(PMe3)3 complex that we discussed earlier for another CO substitution. To initiate the ETC you can either oxidize the complex to [Cr(CO)3(PMe3)3]+ (17e-, half-empty orbital) or reduce it to [Cr(CO)3(PMe3)3] (forming a 19e- complex that would want to dissociate a ligand). Only one of these would be likely to substitute off a CO ligand to replace it with a PMe3 ligand. Which one would you use and why?