Technological Advancements in Molecular Diagnostics is Driving the Growth of this Market
North America Molecular Diagnostics Market by Product (Reagents & Kits, Systems, Software), Test Type (Lab, PoC), Technology (PCR, ISH, INAAT, Sequencing, Microarray), Application (Infectious Diseases, Oncology, Neurological), End User
Download Presentation
Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
Presentation Transcript
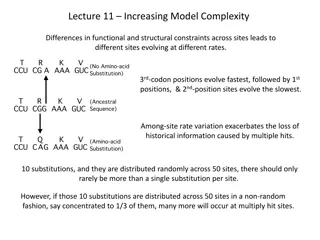
Technological Diagnostics is Driving the Growth of this Market Advancements in Molecular Molecular diagnostics help detect the targeted genetic material in human, viral, and bacterial genomes. Molecular diagnostic tests are increasingly being used in many areas, including testing infectious diseases, oncology, and clinical genetics. Advancements in molecular diagnostics will continue to improve the accuracy and speed of diagnosis and will become an essential aspect of patient-tailored interventions and therapeutics. Polymerase chain reaction (PCR) and microarray technology offer high throughput and more accurate tests. Additionally, sequencing has emerged as an alternative molecular diagnostic technology with diverse applications. The growth of the North America molecular diagnostics market is driven by several factors, including the rising geriatric population, the increasing prevalence of communicable technological advancements in molecular diagnostics, and rising healthcare expenditures. However, unfavorable regulatory frameworks and the high costs of molecular diagnostic tests restrain the growth of this market. & non-communicable diseases, Download Free Sample Now : https://www.meticulousresearch.com/download-sample- report/cp_id=5642?utm_source=pdf&utm_medium=social&utm_campa ign=product&utm_term=28-02-2024 The healthcare landscape is constantly changing due to innovations in medical technologies. In recent years, companies have focused on developing advanced diagnostic kits that yield faster results than traditional kits. These advanced kits allow healthcare providers and caregivers to make crucial medical decisions promptly. For instance, companies have been developing ultra-fast PCR kits to minimize the time required to generate test results. Moreover, traditional PCR tests have transitioned to incorporate multiplex capabilities, including simultaneously detecting two or more pathogens. Multiplex diagnostic tests provide higher accuracy for critical care, which leads to pathogen-specific treatment. Also, the CLIA-waived molecular PoC platform is widely accepted, as traditional POC tests lack sensitivity. Traditional tests can be replaced by rapid molecular diagnostic tests that are simple, easy to use, and highly accurate. Market players are launching PoC molecular PCR tests. For instance, in June 2021, Thermo Fisher Scientific (U.S.) developed the innovative and portable Accula SARS-CoV-2 Test offering gold-standard Reverse Transcription Polymerase Chain Reaction (RT-PCR) detection of SARS-CoV-2 in a point-of-care format. The
test delivers results in 30 minutes, whereas the conventional RT PCR method takes around 2 3 days to produce results. Browse in depth @ https://www.meticulousresearch.com/product/north-america- molecular-diagnostics-market- 5642?utm_source=pdf&utm_medium=social&utm_campaign=product &utm_term=28-02-2024 Companion molecular diagnostics measure the levels of proteins, genes, or specific mutations to reveal specific and effective therapies for an individual s condition. Companion diagnostics (CDx) are a form of personalized, stratified, and precision medicine that individualizes a patient s treatment. CDx has expanded from oncology drugs to multiple therapeutic areas, and the number of combinations has grown significantly over the years. Furthermore, personalized medicine for infectious diseases helps to orient the molecular management of infections. Molecular microbiology offers technologies that can detect and identify microorganisms rapidly. Determining the pharmacogenetic profiles of patients suffering from infectious diseases provides an additional assessment of the drug metabolizer phenotype or the risk of potential adverse drug interactions. Personalized medicine is thus expected to develop new therapies and new methods for disease detection. Request Free Sample Report: https://www.meticulousresearch.com/request-sample- report/cp_id=5642?utm_source=pdf&utm_medium=social&utm_campa ign=product&utm_term=28-02-2024 Key Findings of the Market Study: Based on product & service, the North America molecular diagnostics market is segmented into kits & reagents, instruments, and software & services. In 2023, the kits & reagents segment is expected to account for the largest share of the market. The large market share of this segment is attributed to factors such as the commercial availability of a diverse range of diagnostic reagents & consumables, the availability of disease-specific test kits & assays, growing awareness regarding early disease diagnosis, and the outbreak of COVID-19 Based on technology, the North America molecular diagnostics market is segmented into polymerase chain reaction (PCR), in situ hybridization (ISH), isothermal nucleic acid amplification microarrays, mass spectrometry, sequencing, and other technologies. The sequencing segment is expected to register the highest CAGR during the forecast period. technology (INAAT), Based on test type, the North America molecular diagnostics market is segmented into laboratory tests and PoC tests. In 2023, the laboratory tests
segment is expected to account for the largest share of the market. The large market share of this segment is attributed to the wide availability of laboratory tests in hospitals, diagnostic laboratories, academic institutions, and research centers. Furthermore, laboratory tests are often the preferred choice among patients for diagnostic purposes. Companies efforts to launch new solutions for laboratory tests also contribute to the segment s growth. Quick Buy @ https://www.meticulousresearch.com/Checkout/62458641?utm_sourc e=pdf&utm_medium=social&utm_campaign=product&utm_term=28- 02-2024 Based on end user, the North America molecular diagnostics market is segmented into hospitals & clinics, diagnostic laboratories, academic & research institutes, and other end users. The large market share of this segment is attributed to the increased number of hospitalizations due to various diseases requiring molecular diagnosis and the proliferation of hospitals and clinics in emerging countries, leading to growth in the utilization of molecular diagnostic products. Key Players The key players operating in the North America molecular diagnostics market are Hologic, Inc. (U.S.), Danaher Corporation (U.S.), BioM rieux S.A. (France), Becton, Dickinson and Company (U.S.), Siemens Healthineers AG (Germany), DiaSorin S.p.A. (Italy), Abbott Laboratories (U.S.), Thermo Fisher Scientific Inc. (U.S.), Agilent Technologies, Inc. (U.S.), Illumina, Inc. (U.S.), F. Hoffmann-La Roche (Switzerland), QIAGEN N.V. (Netherlands), and Seegene, Inc. (South Korea). Contact: Mr. Khushal Bombe Meticulous Market Research Inc. 1267 Willis St, Ste 200 Redding, California, 96001, U.S. USA: +1-646-781-8004 Europe : +44-203-868-8738 APAC: +91 744-7780008 Email- sales@meticulousresearch.com Visit Our Website: https://www.meticulousresearch.com/ Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research