Understanding the Nature and Characteristics of Matter
Matter is anything with mass and volume, categorized as solid, liquid, or gas based on physical properties. It consists of tiny particles with intermolecular spaces that are in constant motion and have the ability to diffuse and attract each other. Diffusion and osmosis are processes highlighting movement of molecules, while states of matter exhibit specific characteristics like shape, volume, density, and fluidity based on their state.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
MATTER IN OUR MATTER IN OUR SURROUNDINGS SURROUNDINGS
What is matter? What is matter? Anything that has mass and volume is called matter. Or Anything that has mass and occupies space and can be felt by one or more sense organs is called matter. SI unit of mass is kg and volume is cubic metre (m3 ).
CLASSIFICATION OF MATTER CLASSIFICATION OF MATTER On the basis of physical properties matter is classified into solid, liquid and gas. On the basis of chemical properties matter is classified into element, compound and mixture.
PHYSICAL NATURE OF MATTER PHYSICAL NATURE OF MATTER Every matter is made up of certain particles which differ in shape, size and nature, which differs from other state of matter. Particles of matter are very small/tiny (beyond our imagination).
CHARACTERISTICS OF PARTICLES OF MATTER CHARACTERISTICS OF PARTICLES OF MATTER 1)Particles of matter have space between them called as intermolecular space. 2)Particles of matter are in state of continuous state of motion. It means they possess some kinetic energy. When temperature increase, kinetic energy also increases and hence molecules move faster then. 3)Particles of matter have tendency to diffuse.i.e. to intermix on their own with each other. They do so by getting into spaces between the particles. The intermixing of particles of two different states of matter on their own is called diffusion. 4)Particles of matter attract each other. A force of attraction exists between particles of matter which is called intermolecular force of attraction.
DIFFUSION AND OSMOSIS DIFFUSION AND OSMOSIS Diffusion is a process in which molecules of a substance moves from region of higher concentration to lower concentration and process goes on till uniform mixture is formed. In osmosis, the solvent molecules move from region of low concentration to high concentration through semi-permeable membrane.
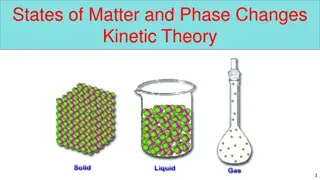
STATES OF MATTER STATES OF MATTER Characteristics Solid state Liquid state Gaseous state Shape Definite shape Indefinite shape Indefinite shape Volume Rigidity Compressibility Definite volume Rigid Non-compressible Definite volume Non-rigid Non-compressible Indefinite volume Non-rigid Highly compressible Density High density (except ice) Less density than solid Least density Rate of diffusion Do not diffuse easily Diffuse very slowly Diffuse at very fast rate Fluidity Do not tend to flow Tend to flow Tend to flow Intermolecular space Intermolecular spaces are very small Intermolecular space is more than solid Intermolecular space is maximum Intermolecular force of attraction Intermolecular force of attraction is maximum Intermolecular force of attraction is less than solids Intermolecular force of attraction is minimum
INTER CONVERSION OF STATES OF MATTER INTER CONVERSION OF STATES OF MATTER The phenomenon of change of matter from one state to another and back to original state by altering the conditions of temperature and pressure is called Inter Conversion of States of Matter.
Melting (Fusion) & Melting Point Melting (Fusion) & Melting Point The process of conversion of a matter from its solid state to its liquid state at a specific condition of temperature and pressure is called fusion/melting. The definite temperature at which a solid start melting is called melting point of that solid. e.g. melting point of ice is 00celsius or 273.16K. Higher the melting point of a substance, greater will be the force of attraction between the particles.
Boiling And Boiling Point Boiling And Boiling Point The process of conversion of a matter from its liquid state to vapors(gaseous state) at specific condition of temperature and pressure is called boiling. It is a bulk phenomenon. The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
Sublimation Sublimation The process of change of solid state directly into gaseous state without passing through the liquid state upon heating and vice-versa on cooling is known as sublimation. e.g. naphthalene, camphor, iodine, ammonium chloride etc.
Vapourization Vapourization The process of conversion of matter from its liquid state to gaseous state at specific conditions of temperature and pressure is called vaporization.
Freezing And Freezing Point Freezing And Freezing Point The process of conversion of matter from its liquid state to its solid at specific condition of temperature and pressure is called freezing. It is the reverse process of fusion/melting. And the definite temperature at which a liquid change into solid state by giving out heat energy at 1 atm is called the freezing point.
Condensation Condensation The process of conversion of matter from its gaseous state to liquid state at specific condition of temperature and pressure is called condensation. It is a reverse process of vaporization.
Effect Of Change Of Temperature Effect Of Change Of Temperature can be concluded as: -
SCALES OF MEASURING TEMPERATURE SCALES OF MEASURING TEMPERATURE Three scales of measuring temperature are as follows: - Temperature on Kelvin Scale = Temperature on Celsius scale +273.16 T(K) =t(oC)+273.16 *Kelvin is the SI unit of temperature, 0oC=273.16K For convenience we write 273 K. Temperature on Celsius Scale = Temperature on Kelvin scale - 273.16 t(oC)=T(K) - 273.16 Temperature on Fahrenheit Scale oF = 9/5 (oC) +32
LATENT HEAT LATENT HEAT The word latent means hidden heat . The heat energy which has to be supplied to change the state of a substance is called its latent heat. Normally temperature of a substance rises when heat is provided to it. But it does not happen when change in state of a substance takes place. Every substance has some force of attraction between its particles which holds them together. If a substance has to change its state, then it is necessary to overcome (or break) these forces of attraction between its particles. The latent heat which we supply is used up in overcoming the forces of attraction between the particles of a substance during its change in state.
EFFECT OF CHANGE OF PRESSURE EFFECT OF CHANGE OF PRESSURE The physical state of a substance can also be changed by changing the pressure. An increase in pressure brings the particles closer and increases the force of attraction between them. e.g when high pressure is applied to gas and its temperature is reduced, the gas is converted to a liquid.i.e. the gas is liquefied.
Intermolecular space reduces after applying pressure. LPG (Liquified Petroleum Gas) cylinder used in our home for cooking or the oxygen supplied to hospitals in cylinders is compressed gas. CNG (Compressed Natural Gas) is used as fuel these days in vehicles. Due to its high compressibility large volumes of gas can be compressed into small cylinders and transported easily.
EVAPORATION EVAPORATION The process of conversion of a liquid into its vapour state at any temperature below its boiling point is called evaporation. The particles present at surface possess comparatively higher kinetic energy as compared to those present in the bulk. Therefore, particles at the surface with higher kinetic energy is able to break away from the force of attraction of other particles and get converted into vapor.
Factors Affecting Evaporation Factors Affecting Evaporation 1)Surface area- Evaporation is surface phenomenon. With increase in surface area rate of evaporation increases and vice- versa. 2)Temperature- with increase in temperature, molecules gain kinetic energy to move faster, so rate of evaporation increases and vice-versa. 3)Humidity-humidity is amount of water vapor present in air. The air around us cannot hold more than a definite amount of water vapor at a given temperature. That is why, clothes dry up faster on a dry day than on a wet day. 4)Wind speed-clothes dry up faster on a windy day because with increase in wind speed particles of water vapor move away with wind decreasing the amount of water vapor in surroundings. So, rate of evaporation increases with the wind speed.