Physical and Chemical Properties of Metals and Non-metals
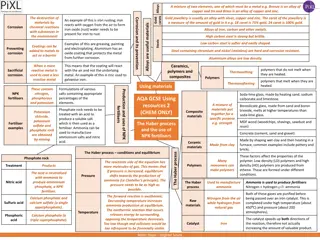
Metals exhibit physical properties like lustrous appearance, ductility, malleability, and sound production. They are also good conductors of heat and electricity. Non-metals, on the other hand, are brittle, non-sonorous, and poor conductors of heat and electricity. The chemical properties include reactions with oxygen forming oxides. Examples of non-metals include sulphur, carbon, and oxygen, while metals like iron rust when exposed to oxygen.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
MATERIALS Types of materials: Metals and non metals. Physical properties of metals: Metals are lustrous, sonorous, malleable, ductile, good conductors of heat and electricty.
LUSTROUS Bright appearance. Ductility: Metals can be drawn in to wires. Sonorous: Producing sound.
MALLEABILITY Metals are lustrous, sonorous, malleable, ductile, good conductors of heat and Ductility: Metals can be drawn in to wires. Sonorous: Producing sound.
GOOD CONDUCTORS Good conductors: Conduct heat and electricity. Hard Examples; Iron, copper, aluminum, magnesium etc
PHYSICAL PROPERTIES Non metals Non-sonorous Brittle Poor conductors of heat and electricity. Non malleable.
NON METALS They breakdown easily. They are not sonorous. They are poor conductors of heat and electricity.
EXAMPLES Non- metals sulphur, carbon, oxygen, phosphorous etc. Exceptions: Metals like sodium, potassium can Cut with knife. Mercury only metal in liquid state.
CHEMICAL PROPERTIES Rusting : Iron reacts with oxygen to form rust. Reaction of metals with oxygen. Metals react with oxygen to form its oxide.
REACTION OF NON METALS WITH OXYGEN Sulphur +oxygen sulphur di-oxide When sulphur di-oxide is added with water, sulphurous acid is formed. It turns blue litmus to red which shows that it is acidic in nature.
METAL OXIDE BASIC IN NATURE When magnesium oxide is added with water and tested with red litmus then red turns to blue, Which shows that it is basic in nature. MgO + H2O Mg(OH)2
REACTION WITH OXYGEN Eg: Iron +oxygen+ water iron oxide(rust) Magnesium +oxygen magnesium oxide
SUMMARY Metals are lustrous, malleable, ductile and good conductors Non-metals are brittle, poor conductors, non lustrous etc Metals react with oxygen to form its oxides Metal oxide is basic in nature. Non metal oxides are acidic in nature.
Thank you End of module II/III