Improving Cancer Care Across European Countries with Cross-Border Healthcare Directive Evaluation
The content discusses the mission of ERN PaedCan to reduce cancer cases by providing advice, access to modern diagnostics and treatments, and expertise through Virtual Tumor Boards. It also highlights the European Governance structure, Key Performance Indicators, a Evaluation Study on the Cross-Border Healthcare Directive 2011/24/EU, and Learnings on the directive's evaluation, emphasizing the challenges and benefits of cross-border healthcare for patients with rare and complex conditions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
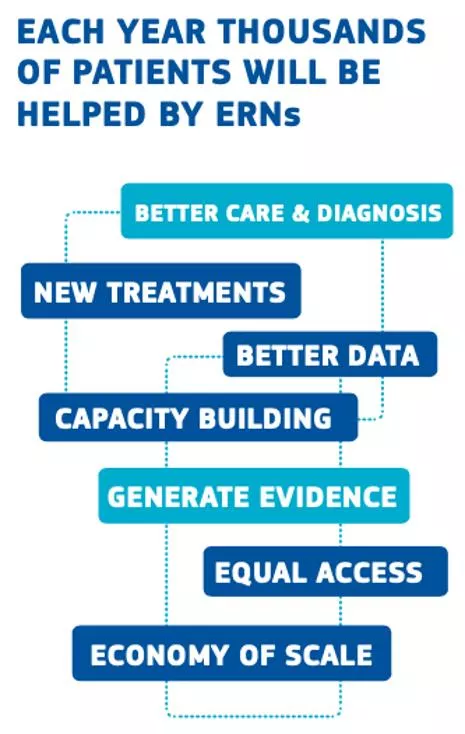
ERN PaedCan Mission Reduce States by enabling advice and access to up-to-date diagnostics and treatments Providing access to a much larger pool of expertise thru Virtual Tumor Boards providing expertise: "Information travels rather than patients." Tackle complex or rare cancer conditions requiring highly specialized interventions. inequalities across Member
ERN PaedCan KPIs ERN PaedCan Number of patients and cross-border referrals, and its proportion by year 19% 23% 10% 9% 13% 16% 9% 14.000 25% 12.000 2.025 20% *FM=Full member, AP= Affiliated Partner 10.000 Number of Patients 15% > 25 European Clinical Standard Practice Guidelines, tight cooperations with ITCC 11301637 8.000 695 842 582 556 6.000 10% 10.393 ERN PaedCan Exchange Programme and Twinning 4.000 5.857 6.315 7.057 7.057 6.146 6.778 Integrated educational activities of SIOPE, ITCC &ERN PaedCan / Educational webinars 5% 2.000 0 0% PancareSurPAss ( Long term follow up), TREL Project (Twinning), EXPeRT PARTNER - Project (VRT), I 2016 9% 556 5.857 2017 13% 842 6.315 2018 16% 1130 7.057 2019 23% 1637 7.057 2020 9% 582 6.146 2021 10% 695 6.778 2022 19% 2.025 10.393 Proportion of CB referrals Cross-border referrals per year: Total number of patients per year Integration of Parent & Survivor Organisation ctn.
Evaluation Study Cross-Border Healthcare Directive 2011/24/EU Implementation - Successes & Barriers Evaluation Study of the Directive 2011/24/EU to ensure patients rights in the EU in cross-border healthcare (SANTE/2021/B2/01) Written by Tetra Tech International Development Sp. z o.o, empirica Communication and Technology Research GmbH, Asterisk Research and Analysis January 2022
Learnings of CBHC 2011/24/EU Evaluation Learnings of CBHC 2011/24/EU Evaluation Martin Dorazil (Deputy Head of Unit, DG Sante 2021) ERNs are effective in supporting the diagnosis and treatment of patients with rare and complex diseases and effectively knowledge (81% agreed) Patients generally preferred to receive care close to home and most were not eager to go abroad even if they could afford it. Patient's rationale for seeking healthcare abroad is based on "need" as much as it is on "choice" System of voluntary prior notification (written confirmation of the estimated amount to be reimbursed)positive, although no definite assurance of cost for patient provided.
Cross-Border Healthcare Directive 2011/24/EU Relevant key barriers for patients' needs According to stakeholders across all sectors Going abroad is difficult because of language barriers , costs associated with travel, no relatives or friends to rely on or no place to stay in outpatient settings. Insufficient information (52%) from HCPs on treatment options in another EU- MS or on relevant medical history (certified translations) for receiving HCP Medical history and NCP need to enhance completeness and accessibility of CBHC related information Difficulties in transferring medical records between systems ad well as challenges in continuity of care in the home country. Financial needs unsolved: 69% identified the need to pay upfront for treatment costs as well as travel costs as main barrier to CBHC) including uncertainty about prices and reimbursements and invoices required by health insurer; The discrepancy in tariffs between countries related to medical services, meaning patients from countries with lower tariffs for services (primarily in Eastern Europe) have to pay the difference from their own pocket if travelling to countries with higher tariffs. Unequal access to innovation - early phase trials often not permitted for CBHC!
Cross-Border Healthcare Directive 2011/24/EU A legal framework for the patient s right to seek healthcare in another Member State and to be reimbursed. for enhanced European cooperation in key areas of healthcare including quality and safety, Health Technology Assessment and eHealth, and rare diseases. Biggest challenge Need for harmonization of interpretations and services across Member States Lack of a transparent evaluation of patients' needs followed by a patient centric approach Biggest questions Timing of CBHC revision and considering a regulation to optimize conditions for patients