WSU Cores Town Hall - Research Infrastructure Events
The WSU Cores Town Hall on February 16, 2021, featured core presentations, breakout rooms, and a platform to increase awareness of WSU's research infrastructure. The event showcased various cores such as Genomics, Proteomics, and more, highlighting their annual revenue and investments to support University Researchers. Detailed order of presentations and contact information for the Animal Model and Therapeutic Evaluation Core were also provided.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Research Cores Town Hall February 16, 2021
Agenda Introductions Drs. Steve Lanier and Tim Stemmler Research Core Presentations Core Directors Breakout Rooms One breakout room for each core to ask questions and obtain more information
Platform to Increase Awareness of WSU Cores WSU Research Infrastructure Events Feb 16 WSU Basic and Translational Science Cores Town Hall March 16 WSU Clinical Research Cores and Resources Town Hall April 20 - WSU Community Based Research Resources Town Hall Starting in late March, OVPR Based Seminars Highlighting 2 Cores/Event OVPR Cores Web Page: https://research.wayne.edu/cores-facilities
WSU Basic and Translational Sciences Cores KCI Cores OVPR Cores Genomics Lipidomics Lumigen Magnetic Resonance Proteomics DLAR Division of Laboratory Animal Research Animal Model & Therapeutics Evaluation Behavioral & Field Research Biobanking & Correlative Sciences Epidemiology Research Microscopy, Imaging & Cytometry Pharmacology & Metabolomics OVPR Cores have an annual revenue of $3.1 million with an overall expenses of ca. $6 million. KCI cores are supplemented by OVPR with a $1 million annual investment. Costs are heavily supplemented to provide affordable support to University Researchers.
Order of Presentations Animal Model & Therapeutic Evaluation Core Lisa Poliin and Sijana Dzinic Behavioral & Field Research Core Felicity Harper & Tanina Moore Genomics Core Katherine Gurdziel Lipidomics Core- Rao Maddipati Lumigen Instrument Center Judy Westrick Magnetic Resonance Core E. Mark Haacke Microscopy, Imaging & Cytometry Resources Core Kamir Moin Pharmacology & Metabolomics Core Jing Li Proteomics Core Paul Stemmer
Animal Model and Therapeutic Evaluation Core Location: 421 E. Canfield 1st Floor Elliman Contact Information: Lisa Anne Polin, Ph.D., Director polinl@karmanos.org or ac8694@wayne.edu Office #1222; Phone (313) 578-4270 Sijana Dzinic, Ph.D., Assistant Director dzinics@karmanos.org or aj6640@wayne.edu Office #1107; Phone (313) 578-4440 Website: https://www.karmanos.org/karmanos/animal-model-and-therapeutics-evaluation-core
Animal Model and Therapeutics Evaluation Core (AMTEC) Mission The purpose of AMTEC is to enhance the peer reviewed funded research activities of Karmanos Cancer Institute members whose research needs primarily involve the use of cancer based animal models and/or preclinical efficacy evaluations of novel or enhanced therapies. Our goal is to provide expert scientific consultation, technical expertise and access to a wide breadth of relevant tumor models and associated animal-related services.
AMTEC Services Collaborates with KCI/WSU investigators to design and conduct tumor biology studies including in vivo efficacy evaluations of novel therapeutics: Study Design and Implementation that include cell/tumor implant, trial set-up and endpoint determinations; dose-range optimization and pilot toxicity studies; administration of novel therapeutic agents; end point data analyses; sample generation, preservation and storage (fresh and snap frozen animal tissues; Advanced Primary and Secondary Evaluations: Combination Therapies, Proof of Principle Designs, etc. Provides investigators with in house derived or purchased mouse and human cell lines or in vivo established tumor models (syngeneic, xenograft, PDX). Performs mouse breeding colony management (transgenic, KO). Provides ad hoc technical assistance including implants, injections, small animal surgical procedures. Assists with preparation of grants, contracts and regulatory filings.
AMTEC New Service AMTEC provides processing of formalin fixed tissues using an Excelsior AS Tissue Processor, with tissue embedding using a HistoStar Instrument Embedding Center with subsequent tissue sectioning available. We can help you further expand your in vivo study findings: Animal study tissue harvest and fixation: Generation of formalin fixed paraffin embedded (FFPE) blocks and slides for follow-up histological examination and pathological analyses. AMTEC service can also provide ad hoc one time training session and SOP to learn principles of H&E staining.
Behavioral and Field Research Core Location: Mid Med Research Building (Same Building as WSU s IRB) Contact Information: Felicity Harper, Ph.D., Scientific Director harperf@karmanos.org Tanina Moore, Ph.D., Director fostert@karmanos.org Website: https://www.karmanos.org/karmanos/behavioral-and-field-science-core
Behavioral and Field Research Core Mission The Behavioral and Field Research Core (BFRC) is a human-based core with a dedicated staff that facilitates the integration of communication and behavioral research. The BFRC s staff offers a wide range of research services and expertise in behavioral research methodologies and comprehensive services spanning the continuum of the research process. The BFRC offers management of evidence-based social and behavioral interventions to support the development and implementation of population science and patient- centered clinical research.
Behavioral and Field Research Core Services Regulatory (PRMC, IRB, Oncore) Clinic recruitment Behavioral study design Survey development (measure selection, cognitive interviewing, pilot testing) Data collection (qualitative and qualitative methods and techniques, e.g. focus groups, in-person and internet-based data collection) Data management and analysis (e.g. survey maintenance, survey and data merge, syntax creation, scale reliabilities)
Genomics Core Location: C.S. Mott Center Ground floor Contact: Katherine Gurdziel, Ph.D., Interim Director gurdziel@wayne.edu or genomicscore@wayne.edu Phone: (313) 577-1002 Website: https://genomesciencescore.wayne.edu/
Genomics Core Mission The Genomics Core offers a streamlined set of services that includes competitive pricing with built-in quality assurance standards. The core has operationalized the latest next-generation sequencing platform Illumina s Nova Seq 6000 and consolidated functional space at the C.S. Mott Center for Human Growth and Development.
Genomics Core Services Provided Next-generation Sequencing (NGS) - NovaSeq 6000 and MiSeq sequencing platforms Library preparation transcriptome profiling, WG-seq (PCR-free), WGBS-seq, exome, 16S Single cell sequencing - 10x Genomics Chromium System employing Next GEM Reagents and Chips Illumina microarray services (human and mouse methylation arrays) SNP genotype analysis - pre-designed and custom TaqMan run on the QuantStudio 12K Flex platform SNP Genotyping Assays TaqMan gene expression analysis run on the QuantStudio 12K Flex platform Gene Expression Assays - real-time Polymerase Chain Reaction (RT-PCR) Bioinformatics support
Lipidomics Core Location: 430 A. Paul Schaap Chemistry Building -5101 Cass Ave. Contact: Krishna Rao Maddipati, Ph.D., Director maddipati@wayne.edu Phone (313) 577- 2088 Yinlong Cai, Ph.D., Lab Manager yinlcai@wayne.edu Phone (313) 577-2088 Website: http://lipidomics.wayne.edu
Lipidomics Core Mission Comprehensive analysis of cellular lipids that encompass fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, sterol lipids, prenol lipids, saccharolipids, and polyketides is in essence the study of the lipid metabolome. Currently, the Lipidomics Core Facility analyzes 5 of the 8 classes of lipids, viz. fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, and sterol lipids on a regular basis and can analyze other classes as well, if needed. In addition, the core facility has developed methods for TCA cycle metabolites and amino acids analyses and gearing up for broader metabolomic analysis.
Lipidomics Core Facility Services Sample preparation: LC-MS analysis of biological samples for lipids requires extraction, either by organic solvents or by solid phase extraction cartridges. Qualitative LC-MS or Shotgun lipid profiling: Evaluation of changing lipid profiles in samples collected under different conditions requires the establishment of the profile. This may involve the establishment of known lipid metabolite profile or identification of novel lipid metabolites, or a combination of both. This service is central to all discovery work. LC-MS method development: Methods are already in place for the detection and quantification of most eicosanoids and docosanoids. However, sample-specific and/or any novel metabolite-specific methods require developmental work. Data analysis: Analysis and presentation of both qualitative and quantitative LC-MS data in the form easily understandable to the investigator as well as interpretation assistance, where necessary.
Lumigen Instrument Center Location: 5101 Cass Ave., A. Paul Schaap Chemistry Building (Basement) Contact: Judy Westrick, Ph.D., Director, judy.westrick@wayne.edu (313) 577-2579 NMR and EPR Laboratory Dennis Anderson, dennis.p.Anderson@wayne.edu Mass Spectrometry Laboratory Johnna Birbeck, Ph.D., jbirbeck@chem.wayne.edu Nick Peraino, Ph.D., nperaino@chem.wayne.edu Electron Microscopy Laboratory Zhi Mei (Mike), Ph.D., zmei@chem.wayne.edu X-Ray Diffraction Laboratory Cassie Ward, Ph.D., Associate Director, ward@wayne.edu Sameera Perera, Ph.D., Samarage.Sameera.Perera@wayne.edu Optical Spectroscopy Laboratory and other Cassie Ward, Ph.D. Associate Director, ward@wayne.edu Website: http://chem.wayne.edu/lumigen/
Lumigen Instrument Center Mission The center supports fundamental and applied research for chemical and material characterization and quantification: NMR and EPR Laboratory Validation (level 1) of synthetic and natural product compounds Assessment of nutrition (metabolomics) Kinetic and equilibrium studies Mass Spectrometry Laboratory Mass Spectrometry Imaging High Resolution Mass Spectrometry, MSn (levels 2, 3, and 4) Trace elements and metals quantification Contaminant quantification in environmental and biological matrices
Lumigen Instrument Center Mission X-Ray Diffraction Laboratory Structural analysis, chemical composition and purity, and physical properties of solid-state materials Electron Microscopy Laboratory High resolution images of materials, cells, and tissues Optical Spectroscopy and other small instruments Thermoanaytical analysis of proteins, polymers, and macromolecular assemblies Quantitation and molecular information
Lumigen Instrument Center Services Usage and fee-for-service core Usage Services Training 24/7 access Instrument fee rate (cost/hour) Fee-for-Service Cost per sample Method development Experimental design Project consultation The Lumigen Instrument Center provides services to the greater Detroit-area and other Michigan scientific communities.
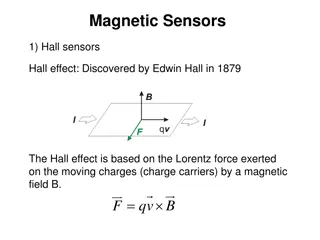
Magnetic Resonance Core Location: MR Research Facility @ Harper University Hospital 3990 John R. Rd. Contact: E. Mark Haacke, Ph.D., Director nmrimaging@aol.com Jiani Hu, Ph.D., Co-Director jianihu@yahoo.com Bruce Berkowitz, 7T Director baberko@med.wayne.edu Zahid Latif Chief MR Technologist zahidlatif@wayne.edu Pavan Jella 3T MR Operator pavan.jella@wayne.edu Robin Roberts 7T MR Operator rroberts@med.wayne.edu (313) 745-1388 Website: http://mrc.wayne.edu
Magnetic Resonance Core Mission The Magnetic Resonance Core is committed to the development of magnetic resonance (MR) methods and their application in preclinical and clinical subjects to better understand human physiology and disease. The MR Research Facility (MRRF) will promote the use of MR methods to the WSU scientific community and support the implementation of MR methods through education, assistance in experimental design, data collection and analysis.
Magnetic Resonance Core Services Magnet time: Pre-clinical Sequence development Protocol development Clinical Data acquisition Research Image processing Developmental* Signal processing *Supported by Office of the Vice President for Research Data analysis
Microscopy, Imaging & Cytometry Resources (MICR) Core Location: MICR-Microscopy: 6339 Scott Hall (313) 577-2511 MICR-Cytometry: 615 HW-CRC (313) 576-8341 MICR-In Vivo Imaging: 0125 Elliman (313) 576-8254 Contact: Kamiar Moin, Ph.D., Director: kmoin@wayne.edu Jessica Back, Ph.D., Associate Director: backj@karmanos.org Website: http://micr.med.wayne.edu/
Microscopy, Imaging & Cytometry Resources Mission The mission of the Microscopy, Imaging & Cytometry Resources (MICR) core is to enhance the peer reviewed funded research activities of investigators microscopy, imaging resources, flow cytometry and related techniques. The facility s objective is to provide the research community with expert scientific consultation, technical expertise and access to state-of-the-art instrumentation. whose research requires
Microscopy, Imaging and Cytometry Resources Core Services Confocal microscopy, multiphoton microscopy, super-resolution microscopy, conventional light microscopy; FRET and FRAP, TIRF and Atomic Force Microscopy; in vivo small animal imaging (including SPECT, CT, PET, X-ray, and optical imaging); Flow Cytometry (including cell sorting, imaging flow cytometry) Ratiometric analyses (e.g., intracellular pH and ion measurement studies), as well as three- and four- dimensional image reconstruction and quantitative measurements; Application and study design consultation; training and workshops.
Pharmacology and Metabolomics Core Location: Karmanos Cancer Institute 4100 John R, HWCRC Room 523 Contact: Jing Li, Ph.D., Director: Lijing@wayne.edu Phone: 313-576-8258 Website: https://karmanos.org/karmanos/pharmacol ogy-core
Pharmacology and Metabolomics Core Mission Provide state-of-the-art bioanalytic technology and a broad range of pharmacology and metabolomics expertise to support basic, translational, and clinical research.
Pharmacology and Metabolomics Core Bioanalysis Services Development, validation, and implementation of liquid chromatography coupled with tandem mass spectrometer (LC-MS/MS) based analytical methods for quantitatively determining drugs, drug metabolites, or endogenous chemicals (metabolites) in biological samples (e.g., plasma/serum, blood, tissue, cell pellets). Waters TQ-XS LC-MS/MS system AB Sciex QTRAP 6500 LC-MS/MS system
LC-MS/MS methods for determination of small molecule drugs and drug metabolites to support clinical or preclinical pharmacokinetic studies # Drugs, Metabolites # Drugs, Metabolites # Drugs, Metabolites acetaminophen 22 dextrophan 44 phenacetin 1 AG127 23 diclofenac, 4 -hydroxy-diclofenac 45 phenylethylamine 2 aminoflavone, AFP464 24 DIM (Diindolmethane) 46 quercetin 3 AZD1775 25 erlotinib 47 quercetin 4 benzylnirvanol 26 estrone 3-sulfate 48 quinidine 5 betulinic acid 27 everolimus 49 ribociclib 6 BGB-290 28 FAU, FMAU 50 RO4929097 7 BMN673 29 fexofenadine 51 rosiglitazone, 5-hydroxy rosiglitazone 8 bupropion, hydroxy bupropion 30 furafylline 52 rosuvastatin 9 carboplatin 31 gefitinib 53 saquinavir 10 CDF (diflourinated-curcumin) 32 HBC (Hydrazinobenzoylcurcumin) 54 sertraline, Venlafaxine 11 ceritinib 33 hydroxyflavone (di, tri, and tetra-) 55 s-mephenytoin, S-4 -hydroxy mephenytoin 12 combretastatin A4 34 irinotecan, SN-38, SN-38G 56 sorafenib 13 Compound #9 (Rad6 inhibitor) 35 isoflavones (Daidzein, Genistein) 57 sulfaphenazole 14 CP-1 36 ketoconazone 58 temozolomide, AIC 15 Cu27 37 lapatinib 59 thiourea 16 CuDDSF2 38 methotrexate, DAMPA 60 thymoquinone 17 curcumin 39 midazolam, 1 -hydroxy midazolam 61 tunicamycin A 18 decetaxel 40 O6-benzylguanine 62 UTL-5g, DCA, ISOX 19 dexamethasone 41 olaparib 63 veliparib, M8 20 dextromethorphan 42 paclitaxel 64 zolmitriptan 21 43 pazopanib
LC-MS/MS based targeted metabolomics for quantitatively profiling ~ 300 endogenous metabolites Number of Metabolites 25 40 60 10 20 15 10 10 8 15 15 65 293 Classes or Pathways Glycolytic, TCA cycle, and pentose phosphate pathway Nucleosides, nucleotides and NAD-related metabolites Amino acid and related metabolites Acyl CoAs Acyl Carnitines Bile acids and metabolites Ceramides Steroids Short chain fatty acids Phospholipids Gut microbial related metabolites Miscellaneous Metabolites Total *Typical metabolites measured are listed above but the capacity is not limited to these. Assays can be tailored to investigators needs.
Pharmacology and Metabolomics Core A broad range of pharmacology support Pharmacokinetic study design to assist study design for pharmacokinetic evaluation in clinical and preclinical studies Pharmacokinetic data analysis and modeling using traditional compartmental or non-compartmental analysis, nonlinear mixed-effect (population) pharmacokinetic modeling, or physiologically based pharmacokinetic modeling approaches In vitro drug metabolism assay to determine metabolic pathways and potential drug-drug interaction In vitro or ex vivo protein binding assay using equilibrium dialysis to determine drug binding to plasma proteins or tissues Permeability and transporter assays using cellular models to determine drug transcellular permeability and interaction with drug transporters
Proteomics Core Location: Scott Hall Room 2105 Contact: Paul Stemmer, Ph.D., Director, pmstemmer@wayne.edu (313) 577-6536 Website: http://research.wayne.edu/proteomics/
Proteomics Core Mission Provide Mass Spectrometry (MS) Technologies for: Proteome Profiling including Differential Abundance Identify Proteins ID & Quantify Chemical Modifications Targeted Quantitation Bioinformatics Molecular Interaction Measurement by Biolayer Interferometry (BLI) Particle Analysis by Microfluidic Pulse Resistance (MFPR)
Proteomics Core Services Proteome Profiling by LC-MS/MS on Orbitrap Instrument Targeted Quantitative Analysis on a Triple Quadrupole MS system All MS systems are equipped with nanoFlow UHPLC Sample Prep by Alkaline Reversed Phase Spin columns Sample Prep by Affinity Selection by antibody for selected proteins or TiO2 for phosphopeptides Peptide and protein identification and quantification using Proteome Discoverer, MaxQuant or PEAKS are performed as part of the MS analysis service Informatics Services including Ingenuity Pathway Analysis (IPA) Molecular interaction analysis by Biolayer Interferometry (BLI) on an Octet Red96 Particle analysis using a Spectradyne nCS1 particle analyzer All services and instrument reservations through the Infinity