Understanding the Degumming Process in Silk Production
Silk is a natural protein fiber composed mainly of fibroin and produced by certain insect larvae. The chemical composition includes fibroin, sericin, fat, wax, and mineral salt. Sericin removal, known as degumming, is crucial for silk quality and involves processes like extraction with water, boil-off in soap, degumming with alkalies, acids, enzymes, or organic amines. Efficient degumming enhances silk's sheen, color, and texture, preparing it for dyeing or weaving. The commercial silk-making process is complex and labor-intensive due to the need to remove impurities like sericin.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
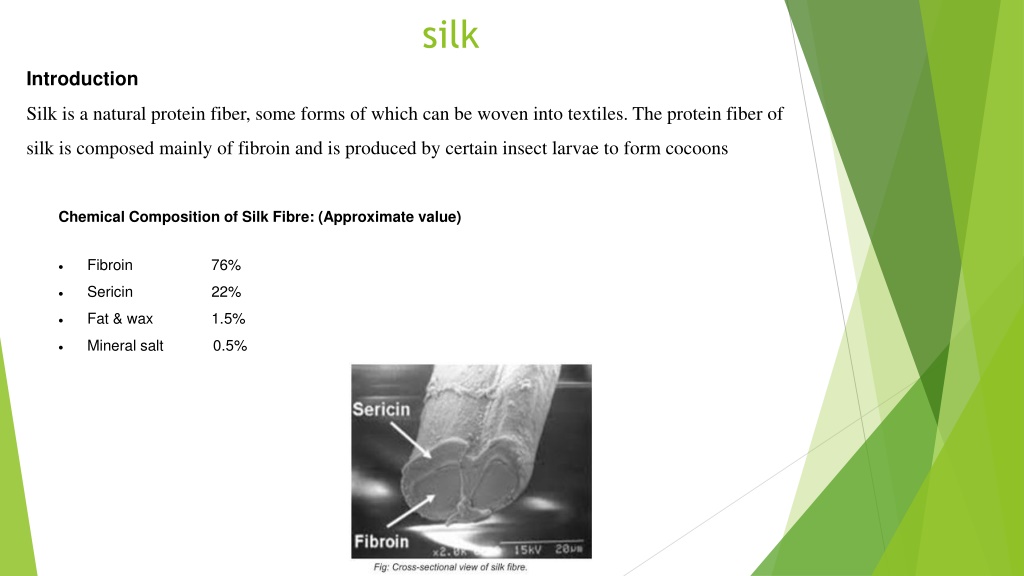
silk Introduction Silk is a natural protein fiber, some forms of which can be woven into textiles. The protein fiber of silk is composed mainly of fibroin and is produced by certain insect larvae to form cocoons Chemical Composition of Silk Fibre: (Approximate value) Fibroin 76% Sericin 22% Fat & wax 1.5% Mineral salt 0.5%
Silk fibre contains approximately 20-25 percent Sericin gum. Sericin is a group of soluble glycoproteins. Silk emitted by the silkworm consists mainly of two proteins, sericin and fibroin; fibroin being the structural center of the silk, and sericin being the gum coating the fibres and allowing them to stick to each other. The chemical composition of sericin is C30H40N10O16. Although both fibroin and sericin are proteins, they differ significantly in their constitution. The commercial process of silk making is highly complex and labor intensive. The following will provide basic information on how silk is pretreated. 1. Degumming/Scouring 2. Bleaching.
1- Degumming/ Scouring A major undesirable constituent part of silk is silk gum or sericin about 25% of total mass. Sericin is removed by degumming. Degumming is the process of removing the sericin, or silk gum, from silk. Removing the gum improves the sheen, color, hand, and texture of the silk. Because the gum can serve as a protective layer, it is typically left on the silk until it is ready to dye. In some cases, the fabric is woven to completion, and then degummed, to protect the yarn from abrasion on the loom. The process involved to remove these impurities is called degumming or scouring of silk. Sericin is insoluble in water. It is comparatively easily hydrolyzed, whereby the long protein molecule of sericin, is broken down into smaller fractions, which are easily dispersed or solubillsed in hot water.
Following are the main Degumming Processes: a) Extraction with water. b) Boil-off in soap. c) Degumming with alkalies. d) Degumming with acids. e) Degumming with enzymes. f) Degumming with organic amines.
Extraction with water Sericin removal can be achieved in water at high temperatures. It is not a commercial process. Up to 96% sericin can be removed by extracting it with water at 115 oC for 3 hours.. Boil-off in soap It is an old process and seems to be still favored commercially. Olive oil soap ( Marseille soap) is the most widely used soap. Following is a typical recipe: Marseille soap 20-30 % owf Time 90-120 min Temperature 90-100 C
Since large amounts of soap are used, this method works out to be expensive. There have been attempts to first carry out partial degumming with a spent or exhausted soap bath and complete the degumming in a fresh bath. Alternately, addition of a small amount of alkali may result in saving of both time and cost. Addition of up to 0.18% of free alkali does not result in weakening of silk. However, excessive alkali in degumming liquor may result in loss of strength, lustre and elasticity of silk. The amount of sericin removal also depends on the type of soap used in the degumming process.
Degumming with Alkali Buffer Although use of alkalis for degumming works out to be economical as compared to soap degumming in terms of chemicals and labour cost, improved productivity etc., it can leave silk yellowish and harsh. Hence alkalis are not recommended to be used alone. With alkalis, the pH of the boiling bath should be maintained between 9.5-10.5. Below pH 9.5, the rate of degumming is too slow and above 10.5, the risk of chemical damage is high. Hence many times, alkaline buffers like sodium carbonate/sodium bicarbonate, sodium hydrogen phosphate/trisodium phosphate and potassium tetraborate/boric acid have been used. Sodium carbonate/sodium bicarbonate is the most widely used buffer.
In addition to the buffer, rate of degumming also depends on concentration of electrolytes in degumming bath. The rate increases with increasing alkali concentration. At a pH of 10 and temperature of 95 oC, the degumming takes 60 min with 0.01 molar Sodium carbonate/sodium bicarbonate concentration but at same pH and temperature, is completed in 20 min with of 0.05 molar alkali concentration. A typical recipe is given below: Sod. Carbonate (0.5 M) Sod. Bicarbonate (0.05M) Non-ionic Surfactant Temperature Time 5.3 gpl 4.2 gpl 3 gpl 96 C 20 min
Presence of salt especially calcium salts in alkaline degumming liquor increases the degradation of silk. Use of sequestering agents like sodium hydrogen pyrophosphate has been known to reduce this damage. Commercial alkali based degumming formulations generally may contain protective agent for silk, sequestrants, wetting/emulsifying agent, buffer salts, alkali etc. examples are: Miltopan SE (Henkel), P-400 (Hepatex AG), Sopajet L (Indifar), Setafin (Henkel), Idrosolvan S40 (Bozzeto) and Serilan PCS (ROTA).