Understanding Serial Dilutions for Bio/Chem/Pharm Students
Introduction to serial dilutions for biochemistry, chemistry, and pharmacy students. Learn how to calculate solution quantities, determine volumes based on different strengths, and convert concentrations effectively in various scenarios. Practical examples provided to enhance understanding. Useful resource for students seeking clarification on serial dilutions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Serial Dilutions www.kent.ac.uk/student-learning-advisory-service
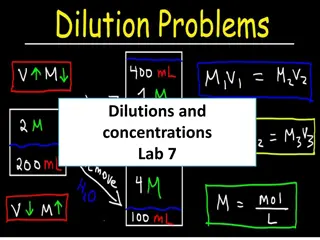
Serial dilutions Serial dilutions Introduction Calculating the quantity of a solution that contains the same amount of substance as different strength solution Bio/chem/pharm students
Serial dilutions Serial dilutions What volume of a 10% v/v solution contains the same amount of ingredient as 200mL of a 25% v/v solution? 1). Use C1V1 = C2V2 percentages cancel percentages cancel 25% 200?? = 10% ? ?? transpose and solve transpose and solve ? =25 200 = 500?? 10
Serial dilutions Serial dilutions Or What you want, over what you have What volume of a 80% v/v solution contains the same amount of ingredient as 240mL of a 5% v/v solution? 1). What you want, over what you have 5 240 80 = 15??
Serial dilutions Serial dilutions A formula asks for 1.6L of a 15% v/v solution. You only have 40% v/v solution. How much should you use? Answer: 600??
Serial dilutions Serial dilutions What volume of a 1 part in 500 w/v solution contains the same amount of ingredient as a 200mL of a 6mg/5mL solution? convert both to percentages convert both to percentages 0.006? 5?? 0.12? 100?? = 0.12% 500=0.2 1 & = 100 = 0.2% then then 0.12 200?? 0.2 = 120??
Serial dilutions Serial dilutions What volume of a 40mg/L solution contains the same amount of ingredient as 10mL of a 0.002% w/v solution? Answer: 5??
To book a maths/stats appointment To book a maths/stats appointment www.kent.ac.uk/student-learning-advisory-service