Understanding Pure Substances, Formulations, and Chromatography in Chemistry
Pure substances are single elements or compounds that have specific properties like color flames and melting points. Formulations are purposefully designed mixtures, while chromatography helps identify ions and separate compounds. Techniques like flame tests and instrumental methods play a crucial role in chemical analysis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
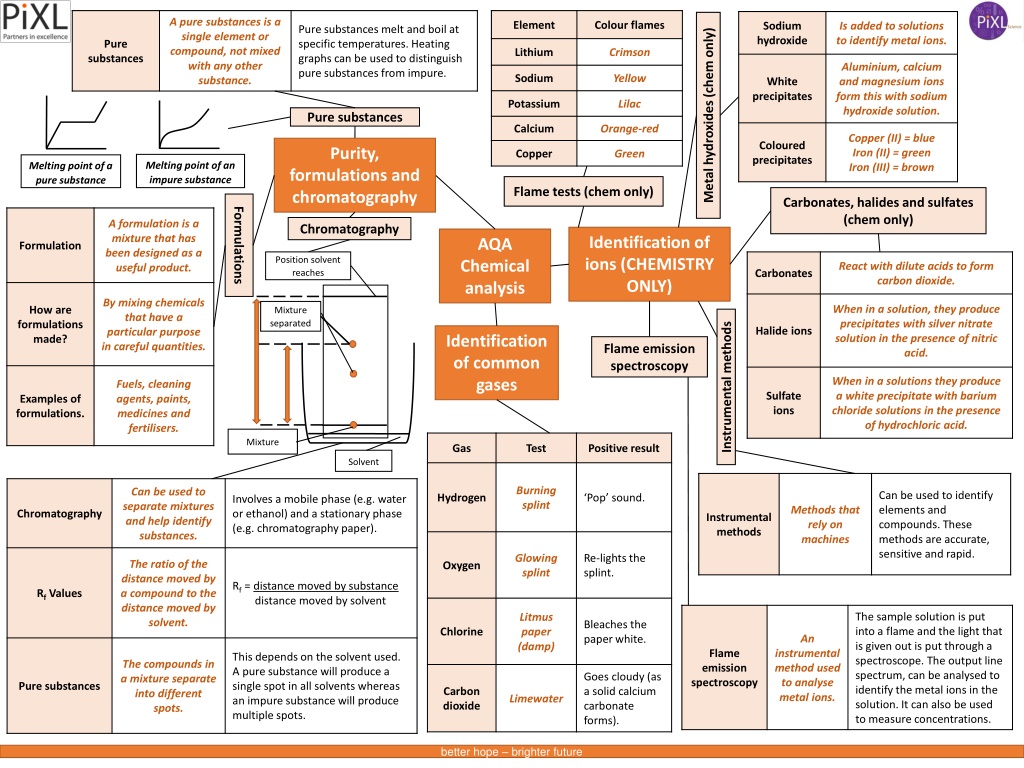
A pure substances is a single element or compound, not mixed with any other substance. Element Colour flames Sodium hydroxide Is added to solutions to identify metal ions. Pure substances melt and boil at specific temperatures. Heating graphs can be used to distinguish pure substances from impure. Metal hydroxides (chem only) Pure Lithium Crimson substances Aluminium, calcium and magnesium ions form this with sodium hydroxide solution. Sodium Yellow White precipitates Potassium Lilac Pure substances Calcium Orange-red Copper (II) = blue Iron (II) = green Iron (III) = brown Coloured precipitates Purity, Copper Green Melting point of an impure substance Melting point of a pure substance formulations and chromatography Flame tests (chem only) Carbonates, halides and sulfates (chem only) Formulations A formulation is a mixture that has been designed as a useful product. Chromatography Identification of ions (CHEMISTRY ONLY) AQA Chemical analysis Formulation Position solvent reaches React with dilute acids to form carbon dioxide. Carbonates By mixing chemicals that have a particular purpose in careful quantities. When in a solution, they produce precipitates with silver nitrate solution in the presence of nitric acid. How are formulations made? Mixture separated Instrumental methods Halide ions Identification of common gases Flame emission spectroscopy When in a solutions they produce a white precipitate with barium chloride solutions in the presence of hydrochloric acid. Fuels, cleaning agents, paints, medicines and fertilisers. Sulfate ions Examples of formulations. Mixture Gas Test Positive result Solvent Burning splint Can be used to separate mixtures and help identify substances. Can be used to identify elements and compounds. These methods are accurate, sensitive and rapid. Hydrogen Pop sound. Involves a mobile phase (e.g. water or ethanol) and a stationary phase (e.g. chromatography paper). Methods that rely on machines Chromatography Instrumental methods Glowing splint Re-lights the splint. The ratio of the distance moved by a compound to the distance moved by solvent. Oxygen Rf = distance moved by substance distance moved by solvent Rf Values The sample solution is put into a flame and the light that is given out is put through a spectroscope. The output line spectrum, can be analysed to identify the metal ions in the solution. It can also be used to measure concentrations. Litmus paper (damp) Bleaches the paper white. Chlorine An Flame emission spectroscopy instrumental method used to analyse metal ions. This depends on the solvent used. A pure substance will produce a single spot in all solvents whereas an impure substance will produce multiple spots. The compounds in a mixture separate into different spots. Goes cloudy (as a solid calcium carbonate forms). Pure substances Carbon dioxide Limewater better hope brighter future
A pure substances is a single element or compound, not mixed with any other substance. Element Colour flames Sodium hydroxide Is added to solutions to identify metal ions. Pure substances melt and boil at specific temperatures. Heating graphs can be used to distinguish pure substances from impure. Metal hydroxides (chem only) Crimson Aluminium, calcium and magnesium ions form this with sodium hydroxide solution. Yellow Lilac Pure substances Orange-red Copper (II) = blue Iron (II) = green Iron (III) = brown Purity, Green Melting point of an impure substance Melting point of a pure substance formulations and chromatography Flame tests (chem only) Carbonates, halides and sulfates (chemistry only) Formulations A formulation is a mixture that has been designed as a useful product. Chromatography Identification of ions (CHEMISTRY ONLY) AQA Chemical analysis Position solvent reaches React with dilute acids to form carbon dioxide. By mixing chemicals that have a particular purpose in careful quantities. When in a solution, they produce precipitates with silver nitrate solution in the presence of nitric acid. Mixture separated Instrumental methods Identification of common gases Flame emission spectroscopy When in a solutions they produce a white precipitate with barium chloride solutions in the presence of hydrochloric acid. Fuels, cleaning agents, paints, medicines and fertilisers. Mixture Gas Test Positive result Solvent Burning splint Can be used to separate mixtures and help identify substances. Can be used to identify elements and compounds. These methods are accurate, sensitive and rapid. Pop sound. Involves a mobile phase (e.g. water or ethanol) and a stationary phase (e.g. chromatography paper). Methods that rely on machines Glowing splint Re-lights the splint. The ratio of the distance moved by a compound to the distance moved by solvent. Rf = distance moved by substance distance moved by solvent The sample solution is put into a flame and the light that is given out is put through a spectroscope. The output line spectrum, can be analysed to identify the metal ions in the solution. It can also be used to measure concentrations. Litmus paper (damp) Bleaches the paper white. An instrumental method used to analyse metal ions. This depends on the solvent used. A pure substance will produce a single spot in all solvents whereas an impure substance will produce multiple spots. The compounds in a mixture separate into different spots. Goes cloudy (as a solid calcium carbonate forms). Limewater better hope brighter future
Element Colour flames Sodium hydroxide Pure substances melt and boil at specific temperatures. Heating graphs can be used to distinguish pure substances from impure. Metal hydroxides (chem only) Pure Lithium substances Sodium White precipitates Potassium Pure substances Calcium Coloured precipitates Purity, Copper Melting point of an impure substance Melting point of a pure substance formulations and chromatography Flame tests (chem only) Carbonates, halides and sulfates (chem only) Formulations Chromatography Identification of ions (CHEMISTRY ONLY) AQA Chemical analysis Formulation Position solvent reaches Carbonates How are formulations made? Mixture separated Instrumental methods Halide ions Identification of common gases Flame emission spectroscopy Sulfate ions Examples of formulations. Mixture Gas Test Positive result Solvent Can be used to identify elements and compounds. These methods are accurate, sensitive and rapid. Pop sound. Involves a mobile phase (e.g. water or ethanol) and a stationary phase (e.g. chromatography paper). Chromatography Instrumental methods Re-lights the splint. Rf = distance moved by substance distance moved by solvent Rf Values The sample solution is put into a flame and the light that is given out is put through a spectroscope. The output line spectrum, can be analysed to identify the metal ions in the solution. It can also be used to measure concentrations. Bleaches the paper white. Flame emission spectroscopy This depends on the solvent used. A pure substance will produce a single spot in all solvents whereas an impure substance will produce multiple spots. Goes cloudy (as a solid calcium carbonate forms). Pure substances better hope brighter future
Element Colour flames Sodium hydroxide Metal hydroxides (chem only) Pure substances White precipitates Pure substances Coloured precipitates Purity, Melting point of an impure substance Melting point of a pure substance formulations and chromatography Flame tests (chem only) Carbonates, halides and sulfates (chem only) Formulations Chromatography Identification of ions (CHEMISTRY ONLY) AQA Chemical analysis Formulation Position solvent reaches Carbonates How are formulations made? Mixture separated Instrumental methods Halide ions Identification of common gases Flame emission spectroscopy Sulfate ions Examples of formulations. Mixture Gas Test Positive result Solvent Chromatography Instrumental methods Rf Values Flame emission spectroscopy Pure substances better hope brighter future