Gas Chromatography: Introduction, Theory, Instrumentation, Derivatization
Gas chromatography is a powerful analytical technique used for separating and analyzing volatile compounds. It involves a mobile gas phase passing through a stationary phase, with components in the mixture interacting differently, resulting in separation. The technique dates back to 1905 and has evolved with advancements in instrumentation, such as gas chromatographs with filters, traps, tanks, columns, injection ports, and detectors. Gas chromatography offers advantages like high sensitivity and speed but has limitations like challenges in reproducibility and surface effects. Various applications include analysis of volatile substances, identification of unknown compounds, and more.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
7thSemester Unit IV Gas Chromatography Introduction, theory, instrumentation, derivatization, Temperature programming, advantages, disadvantages and applications Dr. Nisha Sharma, Associate Professor, Pharmacy, C.S.J.M. University 1
INTRODUCTION: GAS CHROMATOGRAPHY Origin of gas chromatography: 1905, W. Ramsey Separated mixture of gases and vapors Used solid adsorbent: activated charcoal. Gas used as mobile phase: introduced in 1952 by James and Martin. The technique was based on a suggestion made 11 years earlier by Martin and Synge on partition chromatography Martin and Synge were presented the Nobel Prize in chemistry in 1952. Used to analyse volatile substances 2
Partition takes place b/w gas & solid or gas & liq. Nature of stationary phase Fixed stat. phase-solid mat. like granular silica/alumina/C.---GSC Fixed phase. Non vol. liq. Held as thin layer on solid support- (diatomacious earth or keisulguhr)-- - GLC GSC- limited application. Difficult to reproduce surface areas, excessive retention of active gases on solid surfaces which reduce available area, tailing of elution peaks. GLC- Most imp. Widely used. Principle: liq. Partition chrom. Mobile phase in gas liq. Chrom. is gas rather than liquid. 3
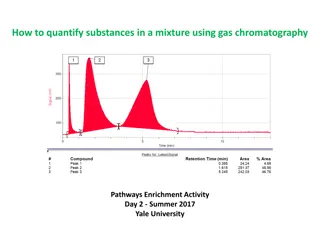
TENTATIVE IDENTIFICATION OF UNKNOWN COMPOUNDS Mixture of known compounds Response Octane Decane 1.6 min = RT Hexane GC Retention Time on Carbowax-20 (min) Response Unknown compound may be Hexane 1.6 min = RT 5 Retention Time on Carbowax-20 (min)
Response Retention Times RT= 4.0 min on SE-30 Hexane GC Retention Time on SE-30 Response RT= 4 min on SE-30 Unknown compound GC Retention Time on SE-30 6
INSTRUMENTATION: Gas Chromatograph Filters/Traps Data system H RESET Regulators Syringe/Sampler gas system inlet column detector data system Inlets Detectors Gas Carrier Hydrogen Air Column Basic Instrumentation: 1. Tank: of carrier gas 3. Column 2. Injection port of sample 4. Detector 7
Carrier gas: He, H, N, Choice of gas-type of detector. Additional regulating valves-for good control of pressure in inlet of column. Gas- inert, available at low cost, should be suitable for detector & type of sample analysed, available in high purity, should not cause risk of fire or explosion hazard. H- dangerous to use, better T.C., Low density, but may react with unsaturated compds & create a fire or explosive hazard. He- 2nd best but explosive, gen used, good T.C. Inert, Low density, great flow rates. N- inexpensive but low sensitivity Air- used only when O in air is useful to the detector or separation. Ex. H or He gives highest sensitivity with TCD because of difference in TC between organic mol. & H/He is greater than other gases 9
Gas cylinder High pressure gas cylinder (gas in compressed) carrier gas reservoir. Pressure regulator- To & control gas flow. Soap bubble meter- To reproduce the rate of carrier gas. Soap film is formed in path of gas when a rubber bulb containing aq sol of soap or detergent if squeezed. Time req for soap film to move b/w 2 graduations on burette is measured & converted to flow rate. 10
Sample Introduction Column inlet-sample port injector. Solute-chrom-Vapor state. Inj port is heated to temp-rapid vaporization, but no thermal degradation of solute Construction of port- Heavy mass, maint at Temp. Sample should be intro immediately into column. Liq/gas sample- inj by syringe-0.1-100 L. Rapid inj. into gas stream. Liq- injected- near- as solutions with syringe-0.5-10 L. Solid- dissolved in suitable solvent-injected as solution. Injection of samples which can t be vaporised at operating temp. are avoided. B cos compds not move appreciably in liq or solid form may clog the port & damage the column. 11
G.C INJECTION SYRINGE: * To rapidly vaporize the sample. * Slow vaporization ses band broadening, by sing the sample plug . * Injection port temperature mostly held 50 C higher than BP of least volatile compd. 12