Understanding Electrophoresis and Its Applications in Biochemistry
Electrophoresis is a vital process in biochemistry that allows for the separation and analysis of charged molecules based on factors such as net charge, size, and electrical field strength. This technique, commonly used in DNA analysis and protein separation, relies on the migration of charged particles in an electric field within a supporting medium. Factors affecting the movement distance in electrophoresis include the net charge on the molecule, size and shape, electrical field strength, supporting medium nature, and electrophoretic temperature. Electrophoresis can be implemented on various charged molecules like amino acids, proteins, DNA, and RNA, providing insights into molecule identification, isolation, and molecular weight determination.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Principle Factors affecting the distance of movement Application Polyacrylamide Gel Electrophoresis (PAGE) Hemoglobin Electrophoresis
Electrophoresis is a process distinguishing and isolating different compounds from each other. It relies on the fact that charged particles (molecules) can migrate in a medium if the medium is subjected to an electrical current.
1 1- - Net charge on the molecule: Particles with negative net charges move toward the anode (positive pole) where as particles with positive charges migrate toward the cathode (negative pole). 2 2- - Size and shape of the molecule: Particles of identical net charge will be distinguished from each other by their size. Heavier molecules will move slower than lighter ones. 3 3- - Strength of the electrical field: The higher the electrical current voltage the further distance travelled and the faster the speed of the movement. 4 4- - Supporting medium physical and chemical nature: Some compounds need special medium, e.g., large polypeptides or proteins are done in polyacrylamide gel where as nucleotide oligomers are done in agarose and polyacrylamide gel. 5 5- - Electrophoretic temperature: Optimal temperature for migration must be used. Net charge on the molecule: Size and shape of the molecule: Strength of the electrical field: Supporting medium physical and chemical nature: Electrophoretic temperature:
Electrophoresis could be implemented on many charged molecules. Such molecules include Amino Acids, Polypeptide Chains, Proteins, nucleotide oligomers, RNA, DNA, Phosphorus sugars and any other ampholytes (molecules whose net charge depends on the pH of the surrounding medium). The medium and voltage power might change from a compound to another depending on the compound chemical nature and size.
1- The identification of certain molecules. 2- The isolation of a certain molecule. 3- The molecular weight of certain molecules.
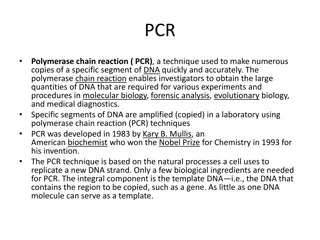
Used routinely in the analysis of single stranded and double stranded DNA. Polyacrylamide is cross linked with TEMED to form a porous gel, thus allowing movement of DNA molecules. Separation of DNA is based on size. For example DNA bands made of 1000-2000 base pairs (bp) can be resolved in 3.5% acrylamide (W/V) where as bands of 6-100 bp are resolved using a 20% acrylamide (W/V). Visualization of the bands could be done by adding a dye such as Ethidium Bromide before or after electrophoresis. Alternatively, radioactively labeled DNA can be visualized by autoradiography (X-ray film).
The gel could be of a denaturing or non- denaturing property. Denaturing polyacrylamide gels are used mainly for sequencing of DNA where as non- denaturing ones are used to detect mutations. An even better and more sensitive technique is "Denaturing-Gradient Gel Electrophoresis". This technique is sensitive enough to detect a single base mutation out of a several hundred long base pairs of DNA.
Principle Hemoglobin (Hgb) migrates according to net charges polypeptide chains. Various types of hemoglobin can be distinguished from one another according to their movements might migrate identically therefore manipulating pH can result in different movements of such hemoglobins (Hgb's). charges of its constituent proteins and movements. Some types of hemoglobin
1 1- - Cellulose Acetate: Performed under alkaline pH = 8.4 8.6. 2 2- - Citrate Agar: Performed under acidic pH = 6.0 6.2. 3 3- - Globin Chain Electrophoresis: Globin chains are separated from Hgb allowing individual electrophoresis of the alpha and non-alpha chains. 4 4- - Isoelecteric The pH of this technique varies according to the constituents of the molecule. It could be between 3 and 10. Cellulose Acetate: Citrate Agar: Globin Chain Electrophoresis: Isoelecteric Focusing (IEF): Focusing (IEF): The first two are used routinely especially in the diagnosis of the common hemoglobin variants (hemoglobinopathies) and Thalassemia. The latter two are used in special cases when the first two techniques could not distinguish the abnormal hemoglobin.