Understanding Solutions and Concentration Units
Explore the world of solutions, the role of solvents and solutes, why solutions form, different concentration units like molarity and molality, calculations involved, colligative properties such as vapor pressure lowering, and how Raoult's Law explains changes in boiling and freezing points in solut
0 views • 13 slides
Understanding Concentration in Solutions
Solutions involve the dissolution of solutes like salt or sugar in solvents such as water, resulting in different concentrations. This concentration can be expressed as moles per liter, known as molarity. By calculating the amount of substance in moles using solution volume and concentration, you ca
0 views • 8 slides
Diluting Solutions in Chemistry
Diluting solutions is a common practice in chemistry to prepare solutions of different concentrations for various experiments and analyses. This involves diluting stock solutions to desired concentrations using the formula M1V1 = M2V2. The process includes preparing calibration curves, determining m
0 views • 6 slides
Preparation of Solution from Solid: Practical General Chemistry Techniques
Solutions are homogeneous mixtures where a solute is dissolved in a solvent. Known concentration solutions can be prepared using solid substances through careful techniques. This process involves weighing the solid, dissolving it in a beaker, transferring to a volumetric flask, and finalizing the so
0 views • 12 slides
Understanding Solution Concentration in Chemistry
Solutions in chemistry are homogeneous mixtures of two or more substances where each maintains its own chemical identity. They consist of a solute, the substance being dissolved, and a solvent, the substance used to dissolve the solute. Solution concentration can be described qualitatively as concen
0 views • 17 slides
Understanding Solution Composition in Chemistry
Exploring the properties of solutions, this content delves into measuring solution composition using techniques like molarity, mass percent, and mole fraction. Through examples, it explains how to calculate the weight percent of solutes in solutions, offering a comprehensive guide on understanding e
0 views • 66 slides
Understanding Solution Concentration and pH Calculations
Explore the concepts of solution concentration, specifically molarity calculations, and pH measurements related to the power of the Hydronium ion. Learn how to calculate molarity, determine the Hydrogen/Hydronium ion concentration, and understand the pH scale from acidic to basic. Practice sample pr
0 views • 13 slides
Chemistry Class Announcements and Demonstrations
Chemistry class announcements include details about an upcoming exam, homework deadlines, and lab submissions. Additionally, demonstrations on solution molarity and concentration are provided to help students understand key concepts. The content also touches on the Flint, Michigan water crisis, illu
0 views • 11 slides
Acid-Base Titration Lab Procedure & Calculations
Conduct an acid-base titration experiment to determine the molarity of an unknown NaOH solution using HCl as the titrant. Follow the outlined steps, record data, perform calculations, and analyze the results to understand the principles of this chemical reaction. Learn about indicators, mole ratios,
0 views • 7 slides
Understanding Solubility Product Constant for Slightly Soluble Salts
Solubility product constant (Ksp) is a special constant that describes the solubility of slightly soluble salts like potassium acid tartrate (KHT) and silver chloride (AgCl) in solution. This experiment aims to determine Ksp for KHT and explore factors affecting Ksp such as temperature and common io
0 views • 13 slides
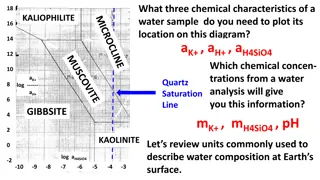
Understanding Chemical Characteristics and Units in Water Composition Analysis
Discover the essential chemical characteristics required to plot a water sample on a diagram and learn about the limitations of using molarity for concentrated solutions of divalent ions, especially when dealing with changing temperatures. Explore the concept of molality and its advantages, while al
0 views • 13 slides
Understanding Molarity and Molality in Solutions
Solutions are combinations of solvent and solute, with varying concentrations affecting their properties. Molarity and molality are key measures of concentration, and saturation levels impact solubility. Explore examples to calculate molarity for solutions in different scenarios.
0 views • 12 slides
Chemical Measurements Overview
In Chapter 1 - Chemical Measurements, you will delve into SI Units, derived SI units, prefixes, unit conversions, concentrations like molarity and molality, and percent compositions. Explore how to use prefixes for calculations, convert units, and understand different concentration measurements for
0 views • 22 slides
Understanding Concentration and Dilution of Solutions by Dr. Laila Al-Harbi
Dr. Laila Al-Harbi explains the concept of concentration of solutions, focusing on molarity and the calculation involved. The molar concentration of solutions is determined by the amount of solute in a given volume of solvent, expressed as moles of solute per liter of solution. Additionally, the pro
0 views • 15 slides
Chemistry Study Materials and Practice Questions
This collection of chemistry study materials includes information on reactions, solubility, electrolytes, and solution stoichiometry. Practice questions cover classifying solutes, predicting solubility of salts, writing ions produced in water, and determining precipitates in reactions. Includes a mo
0 views • 15 slides
Understanding Solution Concentration: Lesson in Chemistry
Explore the concept of solution concentration through an engaging experiment involving gummy bears in sugar and water solutions. Learn about key terms such as solution, solute, solvent, saturated solution, concentration, and molarity. Discover how molecules move based on concentration gradients and
0 views • 10 slides
Understanding Solutions and Concentration in Chemistry
Explore the concepts of solutions, solutes, solvents, concentration, and molarity through engaging activities like the "Bearly Alive" experiment and discussions on saturated solutions. Learn to define and calculate molarity, and understand how compounds behave in solution. Get ready for hands-on lab
0 views • 13 slides
Chemical Substances Preparation Methods
Methods for preparing chemical substances including calculations for molarity, preparation of solutions from solid and liquid substances, and examples for preparing specific solutions like BaCl2 and CuSO4. Explained techniques for calculating the amount of solid substances needed to achieve a desire
0 views • 8 slides