Understanding Atomic Structure and Subatomic Particles
Delve into the world of atomic structure and subatomic particles to reveal the inner workings of elements. Discover how to determine atomic mass, identify protons, neutrons, and electrons, and interpret the periodic table. Explore the key concepts of isotopes, electron configurations, and the characteristics of various elements. Enhance your knowledge of chemistry with a focus on the fundamental components of matter.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Resilience: Use various rules to determine atomic mass and structure Atomic Structure L/O Deduce the structure of different elements Last week: State how you would separate a mixture of a liquid and an insoluble solid Last term: Identify the type of wave found in water Last year: Calculate the actual size of the section of the onion cells Key Words Proton Neutron Election Nucleus Shell
How to Read the periodic table This is Beryllium It s atomic mass is 9 (this biggest number and atomic number is 4 (the smallest number). To draw out the atom like below these numbers are Key To know how many protons and electrons there are you look for the atomic number which in this case is 4. Picture below shows this. Then to work how many neutrons you the atomic number away from the atomic mass which is 9-4= 5 so you now then have 5 neutrons So the answer would be Protons:4 Electrons:4 Neutrons:5
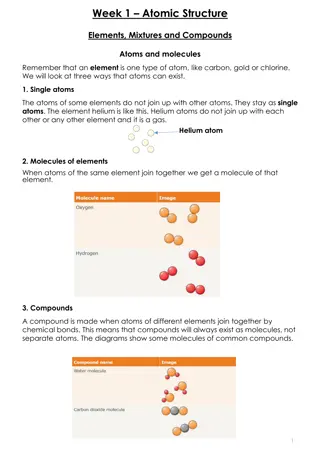
Atoms are made up of: Protons found in the nucleus Neutrons found in the nucleus Electrons found in shells around the outside of the atom
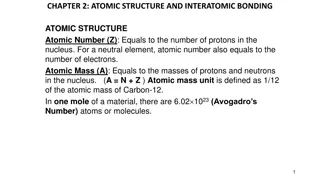
An atom is made up of 3 types of subatomic particle: Protons and neutrons are found in the nucleus Electrons are found around the nucleus in electron shells Atomic number = number of protons Number of protons = number of electrons Atomic mass = number of protons + number of neutrons
Bronze: 1. Deduce the number of subatomic particles in carbon, lead, mercury and radium. 2. Explain why all of these elements have no overall charge. Silver: 1. Explain why a mercury atom does not have an overall charge. Justify whether this is true for all atoms. 2. Explain the difference between the atomic number and mass number for an element. Gold: 1. Produce a set of instructions for somebody to calculate the number of protons, neutrons and electrons in an atom using the periodic table. 2. Justify why a cathode ray tube was able to separate out the subatomic particles of an atom. 3. Explain why the electron was deflected further than the proton
Progress Questions 1. Magnesium contains 12 electrons. It has an electron configuration of: a) 6.6 b) 8.4 c) 2.8.2 2. Use the electron configuration of magnesium to identify which group and period of the periodic table it is found in. 3. Explain what is meant by an isotope.