Exploring Atom Structure and Properties Questions
This series of questions delves into the intriguing world of atoms, covering topics such as atom size comparison, proton count identification, location of metals on the Periodic Table, nucleus charge explanation, atomic number significance, various arrangements of the Periodic Table, subatomic particle characteristics, atomic mass determination, element identification, nucleus charge calculation based on proton, neutron, and electron counts, and understanding reactivity factors within an atom.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Question One Contrast the size of a atom with the size of a flea?
Question 3 If there are 12 protons in an atom, then what atom is it?
Question 4 Explain where the metals are located on The Periodic Table?
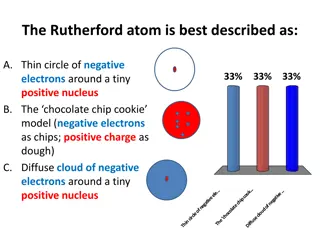
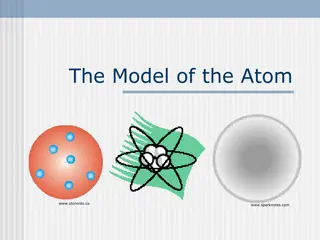
Question 5 This model is a model of what atom?
Question 6 Why is the charge of the nucleus positive?
Question 7 Why is the atomic number of Hydrogen one?
8 List the five different ways that The Periodic Table is arranged.
Question 8 Which of these best describes one of the subatomic particles that could be found at location X in the model of an atom shown above? A. It has mass but no charge. B. It has no mass and a positive charge. C. It has a large mass and a negative charge. D. It has no mass and an equal number of positive and negative charges.
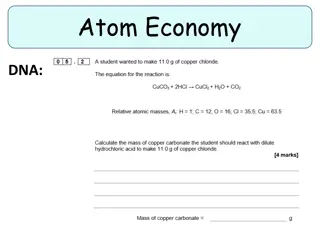
Question 9 What is the Atomic Mass of the atom. Explain your answer.
Question 10 What element does this atom represent?
Question 11 If an atom has 5 protons, 6 neutrons, and 5 electrons, then what is charge of its nucleus?
Question 12 What part of the atom gives it it s reactivity?