Understanding Alloys and Corrosion in Metals
Alloys are mixtures of elements, with one being a metal, like bronze and brass. Gold jewelry is often alloyed with metals like silver and copper. Corrosion, the deterioration of materials through chemical reactions, can be prevented by using protective coatings. Different types of steels and aluminum alloys offer varying degrees of strength and resistance to corrosion. Composite materials, ceramics, and polymers play essential roles in various applications. Learn about the production of fertilizers, composite materials, and the processes involved in manufacturing metals and alloys.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
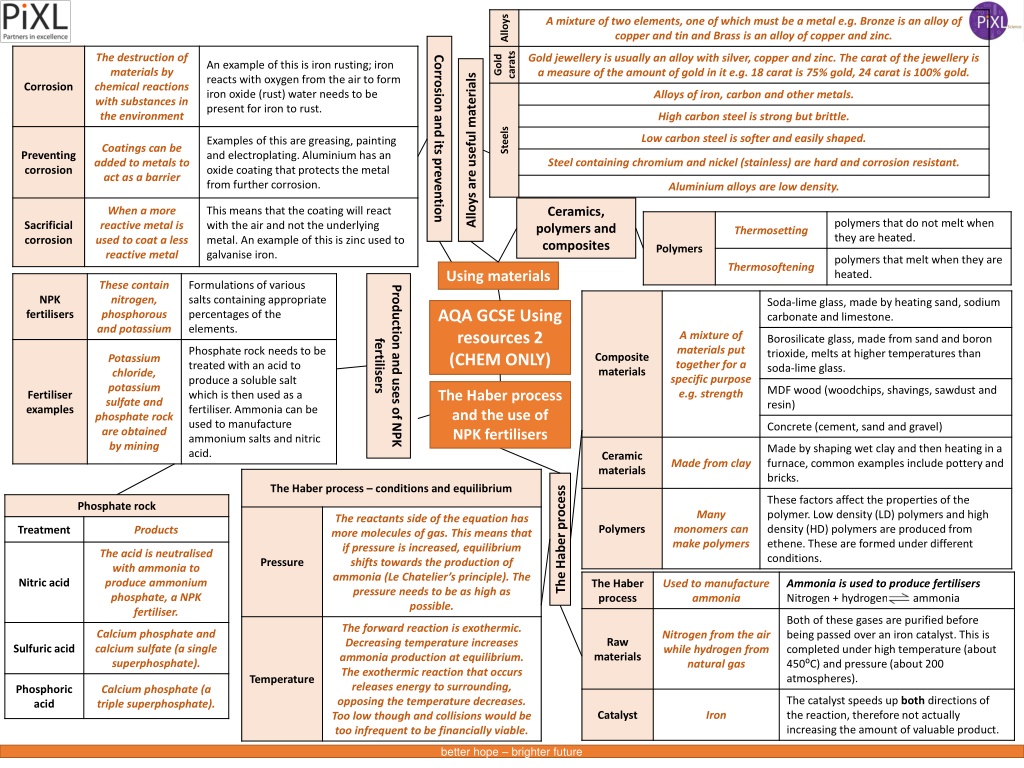
Alloys A mixture of two elements, one of which must be a metal e.g. Bronze is an alloy of copper and tin and Brass is an alloy of copper and zinc. carats Gold The destruction of materials by chemical reactions with substances in the environment Gold jewellery is usually an alloy with silver, copper and zinc. The carat of the jewellery is a measure of the amount of gold in it e.g. 18 carat is 75% gold, 24 carat is 100% gold. Corrosion and its prevention An example of this is iron rusting; iron reacts with oxygen from the air to form iron oxide (rust) water needs to be present for iron to rust. Alloys are useful materials Corrosion Alloys of iron, carbon and other metals. High carbon steel is strong but brittle. Steels Low carbon steel is softer and easily shaped. Examples of this are greasing, painting and electroplating. Aluminium has an oxide coating that protects the metal from further corrosion. Coatings can be added to metals to act as a barrier Preventing corrosion Steel containing chromium and nickel (stainless) are hard and corrosion resistant. Aluminium alloys are low density. Ceramics, polymers and composites When a more reactive metal is used to coat a less reactive metal This means that the coating will react with the air and not the underlying metal. An example of this is zinc used to galvanise iron. polymers that do not melt when they are heated. Sacrificial corrosion Thermosetting Polymers polymers that melt when they are heated. Thermosoftening Using materials These contain nitrogen, phosphorous and potassium Formulations of various salts containing appropriate percentages of the elements. Production and uses of NPK NPK Soda-lime glass, made by heating sand, sodium carbonate and limestone. AQA GCSE Using resources 2 (CHEM ONLY) fertilisers A mixture of materials put together for a specific purpose e.g. strength Borosilicate glass, made from sand and boron trioxide, melts at higher temperatures than soda-lime glass. fertilisers Phosphate rock needs to be treated with an acid to produce a soluble salt which is then used as a fertiliser. Ammonia can be used to manufacture ammonium salts and nitric acid. Composite materials Potassium chloride, potassium sulfate and phosphate rock are obtained by mining MDF wood (woodchips, shavings, sawdust and resin) The Haber process and the use of NPK fertilisers Fertiliser examples Concrete (cement, sand and gravel) Made by shaping wet clay and then heating in a furnace, common examples include pottery and bricks. Ceramic materials Made from clay The Haber process conditions and equilibrium The Haber process These factors affect the properties of the polymer. Low density (LD) polymers and high density (HD) polymers are produced from ethene. These are formed under different conditions. Phosphate rock Many The reactants side of the equation has more molecules of gas. This means that if pressure is increased, equilibrium shifts towards the production of ammonia (Le Chatelier s principle). The pressure needs to be as high as possible. Polymers monomers can make polymers Treatment Products The acid is neutralised with ammonia to produce ammonium phosphate, a NPK fertiliser. Pressure Nitric acid The Haber process Used to manufacture ammonia Ammonia is used to produce fertilisers Nitrogen + hydrogen ammonia Both of these gases are purified before being passed over an iron catalyst. This is completed under high temperature (about 450 C) and pressure (about 200 atmospheres). The forward reaction is exothermic. Decreasing temperature increases ammonia production at equilibrium. The exothermic reaction that occurs releases energy to surrounding, opposing the temperature decreases. Too low though and collisions would be too infrequent to be financially viable. Calcium phosphate and calcium sulfate (a single superphosphate). Nitrogen from the air while hydrogen from natural gas Raw Sulfuric acid materials Temperature Phosphoric acid Calcium phosphate (a triple superphosphate). The catalyst speeds up both directions of the reaction, therefore not actually increasing the amount of valuable product. Catalyst Iron better hope brighter future
A mixture of two elements, one of which must be a metal e.g. Bronze is an alloy of copper and tin and Brass is an alloy of copper and zinc. The destruction of materials by chemical reactions with substances in the environment Gold jewellery is usually an alloy with silver, copper and zinc. The carat of the jewellery is a measure of the amount of gold in it e.g. 18 carat is 75% gold, 24 carat is 100% gold. Corrosion and its prevention An example of this is iron rusting; iron reacts with oxygen from the air to form iron oxide (rust) water needs to be present for iron to rust. Alloys are useful materials Alloys of iron, carbon and other metals. High carbon steel is strong but brittle. Low carbon steel is softer and easily shaped. Examples of this are greasing, painting and electroplating. Aluminium has an oxide coating that protects the metal from further corrosion. Coatings can be added to metals to act as a barrier Steel containing chromium and nickel (stainless) are hard and corrosion resistant. Aluminium alloys are low density. Ceramics, polymers and composites When a more reactive metal is used to coat a less reactive metal This means that the coating will react with the air and not the underlying metal. An example of this is zinc used to galvanise iron. polymers that do not melt when they are heated. Thermosetting Polymers polymers that melt when they are heated. Thermosoftening Using materials These contain nitrogen, phosphorous and potassium Formulations of various salts containing appropriate percentages of the elements. Production and uses of NPK Soda-lime glass, made by heating sand, sodium carbonate and limestone. AQA GCSE Using resources 2 (CHEM ONLY) A mixture of materials put together for a specific purpose e.g. strength Borosilicate glass, made from sand and boron trioxide, melts at higher temperatures than soda-lime glass. fertilisers Phosphate rock needs to be treated with an acid to produce a soluble salt which is then used as a fertiliser. Ammonia can be used to manufacture ammonium salts and nitric acid. Potassium chloride, potassium sulfate and phosphate rock are obtained by mining MDF wood (woodchips, shavings, sawdust and resin) The Haber process and the use of NPK fertilisers Concrete (cement, sand and gravel) Made by shaping wet clay and then heating in a furnace, common examples include pottery and bricks. Made from clay The Haber process conditions and equilibrium The Haber process These factors affect the properties of the polymer. Low density (LD) polymers and high density (HD) polymers are produced from ethene. These are formed under different conditions. Many The reactants side of the equation has more molecules of gas. This means that if pressure is increased, equilibrium shifts towards the production of ammonia (Le Chatelier s principle). The pressure needs to be as high as possible. monomers can make polymers Phosphate rock Treatment Products The acid is neutralised with ammonia to produce ammonium phosphate, a NPK fertiliser. Used to manufacture ammonia Ammonia is used to produce fertilisers Nitrogen + hydrogen ammonia Both of these gases are purified before being passed over an iron catalyst. This is completed under high temperature (about 450 C) and pressure (about 200 atmospheres). The forward reaction is exothermic. Decreasing temperature increases ammonia production at equilibrium. The exothermic reaction that occurs releases energy to surrounding, opposing the temperature decreases. Too low though and collisions would be too infrequent to be financially viable. Nitrogen from the air while hydrogen from natural gas Calcium phosphate and calcium sulfate (a single superphosphate). Calcium phosphate (a triple superphosphate). The catalyst speeds up both directions of the reaction, therefore not actually increasing the amount of valuable product. Iron better hope brighter future
Alloys Corrosion and its prevention carats Gold An example of this is iron rusting; iron reacts with oxygen from the air to form iron oxide (rust) water needs to be present for iron to rust. Alloys are useful materials Corrosion Steels Examples of this are greasing, painting and electroplating. Aluminium has an oxide coating that protects the metal from further corrosion. Preventing corrosion This means that the coating will react with the air and not the underlying metal. An example of this is zinc used to galvanise iron. Ceramics, polymers and composites polymers that do not melt when they are heated. Sacrificial corrosion Polymers polymers that melt when they are heated. Using materials Formulations of various salts containing appropriate percentages of the elements. Production and uses of NPK NPK Soda-lime glass, made by heating sand, sodium carbonate and limestone. AQA GCSE Using resources 2 (CHEM ONLY) fertilisers Borosilicate glass, made from sand and boron trioxide, melts at higher temperatures than soda-lime glass. fertilisers Phosphate rock needs to be treated with an acid to produce a soluble salt which is then used as a fertiliser. Ammonia can be used to manufacture ammonium salts and nitric acid. Composite materials MDF wood (woodchips, shavings, sawdust and resin) The Haber process and the use of NPK fertilisers Fertiliser examples Concrete (cement, sand and gravel) Made by shaping wet clay and then heating in a furnace, common examples include pottery and bricks. Ceramic materials The Haber process conditions and equilibrium The Haber process These factors affect the properties of the polymer. Low density (LD) polymers and high density (HD) polymers are produced from ethene. These are formed under different conditions. Polymers Phosphate rock Treatment Products Pressure The Haber process Ammonia is used to produce fertilisers Nitrogen + hydrogen Nitric acid ammonia Both of these gases are purified before being passed over an iron catalyst. This is completed under high temperature (about 450 C) and pressure (about 200 atmospheres). Raw Sulfuric acid materials Temperature Phosphoric acid The catalyst speeds up both directions of the reaction, therefore not actually increasing the amount of valuable product. Catalyst better hope brighter future
Alloys Corrosion and its prevention carats Gold Alloys are useful materials Corrosion Steels Preventing corrosion Ceramics, polymers and composites Sacrificial corrosion Polymers Using materials Production and uses of NPK AQA GCSE Using resources 2 (CHEM ONLY) NPK fertilisers fertilisers Composite materials The Haber process and the use of NPK fertilisers Fertiliser examples Ceramic materials The Haber process conditions and equilibrium The Haber process Polymers Phosphate rock Treatment Products Pressure The Haber process Raw materials Temperature Catalyst better hope brighter future