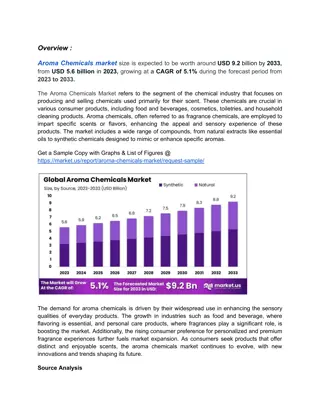
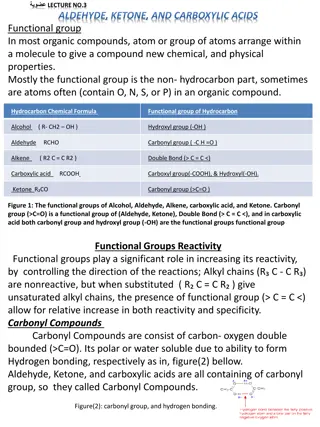
Qualitative Analysis of Aldehydes and Ketones in Chemistry Lab
In this Chemistry 318 lab, students will conduct qualitative analysis of aldehydes and ketones using chemical and spectroscopic methods. The lab includes classification tests, spectroscopy (IR, 1H-NMR/13C-NMR, MS), and identification of unknown compounds. Experimental procedures involve physical evaluation, distillation, refractive index measurements, and qualitative tests for solubility, 2,4-DNP, chromic acid, and Tollens test. Students must record detailed observations and report results to the instructor as per the lab schedule.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
QUALITATIVE ANALYSIS ALDEHYDES & KETONES Chemistry 318 Fall 2018
Schedule of day PPE check at the door Pre-lab check at the door Quiz Recitation Qualitative Analysis: Aldehydes & Ketones Safety Put bags away Goggles Gloves Lab Coat LAB!
Due Dates Today Grignard Reaction Report See instructions on Bb Notebook & Lab Report Formats for Synthesis report. End of lab notebook yellow pages (QA: Aldehydes & Ketones) Next Week At the beginning of lab Qualitative Analysis: Aldehydes and Ketones Report See instructions on Bb Notebook & Lab Report Formats for Identification of Unknowns report.
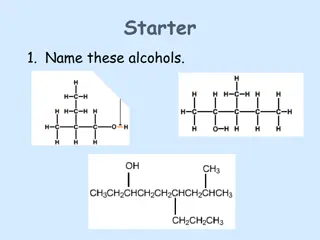
The Experiment Qualitative Analysis Identification and characterization of unknown compounds using chemical and spectroscopic methods. Chemical classification test tube reactions that are fast and give a visual result Spectroscopy IR; 1H-NMR/13C-NMR; MS The unknowns for this lab are either aldehydes or ketones. Today you ll be performing all the chemical tests, take an IR, and receive an NMR.
Experimental Notes 1. Begin with physical evaluation Odor, color, physical state 2. Simple distillation to purify unknown and get B. P. 3. Refractive index remember to record room temperature! Use both the digital and analog refractometers. 4. Perform qualitative tests Record all observations in your notebook; do not merely record positive or negative . Be detailed and specific!
Qualitative Analysis Tests Solubility H2O and H2SO4 Watch for color changes or heat evolution with H2SO4! Positive results indicates a reaction of >C=O with H+ 2,4-DNP [no reagent available!] H+ R R H2N NH NO2 C N NH NO2 C O R' H2O - R' NO2 NO2 2,4-dinitrophenylhydrazine 2,4-dinitrophenylhydrazone Ppt color: Extent of conjugation: unconjugated yellow orange red some highly
Qualitative Analysis Tests Chromic Acid Test Aldehydes are easily oxidized ketones are not R R H+ C O Cr2(SO4)3 C O H2Cr2O7 HO H orange sol n green ppt Tollens Test Aldehydes are easily oxidized ketones are not R Ag(NH3)2OH R Ag0 C O C O NH4 O H Show the results of your test to the instructor. Show the results of your test to the instructor. silver mirror
Qualitative Analysis Tests Iodoform Test (methyl ketones) OH- I2 R R R C O HCI3 C O C O CI3 CH3 NaOH O yellow ppt Use a large test tube! It may take a long time, so do this test first Show the results of your test to the instructor. Show the results of your test to the instructor.
Experimental Notes Each student gets their own unknown compound. You may work in groups (of 2) on the known compounds. One person sets up each test for the knowns and unknowns. Both people perform the tests and observe and interpret results. Both people are responsible for the results. DO NOT divide the work by giving one person sole responsibility for a test. Each student is responsible for their unknown results. Use the known compounds that are listed in the Table in the Lab Manual Unknown possibilities are listed in the appendix of thelab manual
Experimental Notes Unknown possibilities are listed in the appendix of the Lab Manual. All waste goes into the WASTE JAR in the Hood
Experimental Notes When you are finished with your qualitative chemical tests, write a paragraph in your notebook that summarizes the results. You then should be able to justify the conclusion that you have either an aldehyde or a ketone (and if you have a ketone, whether it is a methyl ketone or not). After you have successfully justified your conclusion (orally [(if required by the instructor] and in writing [take to your instructor for approval], you can take an IR. Do NOT take an IR before receiving approval from your instructor. After you have taken your IR, you will receive an NMR.