Structural Determination of Tropine, Atropine, and Tropic Acid
Structural determination of compounds such as Tropine, Atropine, and Tropic Acid involves various tests and synthesis methods. Ester linkages, functional groups, and compound structures are analyzed through experiments like the Muller and Wislicenus synthesis. Different proposals and confirmations regarding the nature of nitrogen, carbon groups, and heterocycles are discussed. The process includes oxidation studies, derivatization, and degradation experiments to accurately determine the structures of these organic compounds.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
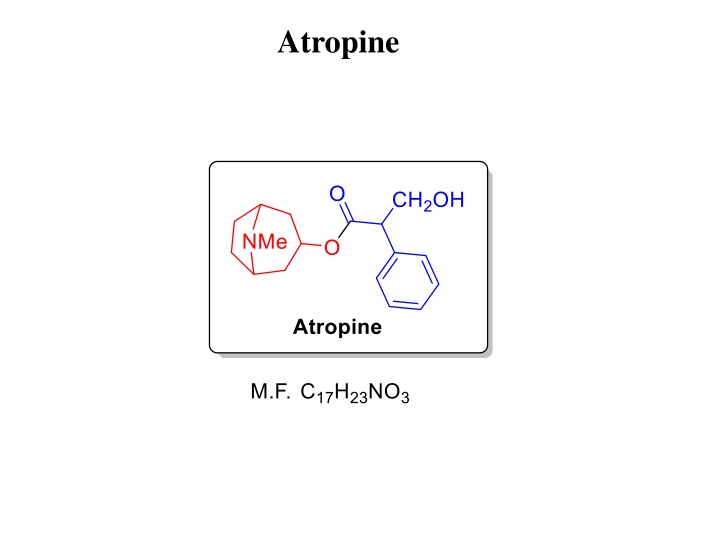
Structure determination of Atropine DBEs = 7 Nature of linkage could be amide or ester!!! Since the nature of nitogen in Tropine was found as tertiary, so there should be an ester linkage.
Structure determination of Tropic acid Functional group test: DBEs = 5 Presence and number of COOH group: Present, 1 number Presence and number of OH group: Present, 1 number Nature of OH group : Primary
Structure determination of Tropic acid Atropic acid was formed by the dehydration of Tropic acid, so the structure of Tropic acid could be either II or IV. Structure IV was confirmed as Tropic acid.
Synthesis of Tropic acid Muller and Wislicenus synthesis
Structure determination of Tropine DBEs = 2 FG test: Nature of nitrogen- tertiary, N-CH3 group present -OH group present, nature of OH- secondary alcohol Ladenburg Based on these observations Ladenburg proposed following two structures of Tropine but both were incorrect as Tropine do not add on the bromine.
Structure determination of Tropine Merling Oxidation (degradation) studies Tropinic acid and Tropine have same number carbons so Merling suggested that -OH group must be present on a ring and he proposed following two saturated structures of Tropine. Willstatter Reinvestigated the oxidation of Tropine Isolation of Tropinone (a ketone) confirmed the nature of OH as secondary. Isolation of N-methylesuccinimide confirmed the presence of 5-membered heterocycle. N-atom must be common to both piperidine (6-memb) as well as pyrrolidine (5-memb) ring since there is only one N-atom in Tropine.
Structure determination of Tropine Willstatter Derivatization of Tropinone confirmed the presence of CH2-CO-CH2- moiety in Tropine. Based on these observations he modified Merlings structure and proposed folloing structure which was found correct by further degradation studies.
Synthesis of Tropine Robinson s synthesis Single step synthesis
Stereochemistry of Tropine Fodor s stereochemistry of Tropine: Proposed boat conformer of Tropine moiety in Atropine Bose s stereochemistry of Tropine: Proposed chair conformer of Tropine moiety in Atropine