Slope and Line Steepness
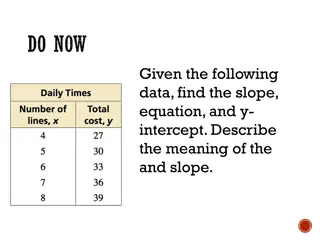
Learn about slope, which is a number indicating line steepness. Positive slopes go up to the right, negative slopes go down, and horizontal lines have a slope of 0. Find slope by ratio of vertical and horizontal changes. Explore examples to practice concepts.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Calibration of SUS pH electrodes
Hamilton SUS pH electrodes Quality Declaration : All sensors delivered with Quality Declaration The Quality Declaration contains all information needed for start-up 2
Hamilton SUS pH electrodes Quality Declaration : Zero point Most equipment (controllers or transmitters) need zero point value and slope The zero point value is the mV value at pH 7, directly provided in the declaration 3
Hamilton SUS pH electrodes Quality Declaration : Slope The slope is calculated based on zero point value and pH 4 reading according to following formula (all mV values at 25 C) : Slope= [E(pH4.01)-E(pH7.00)] / [(pH=4.01) (pH=7.00)] In previous example : Slope = (181 4)/-2.99 = -59.2mV/pH 4
Hamilton SUS pH electrodes Quality Declaration : Zero point as pH value for 0 mV Some equipment may need the slope and instead the zero point, they require the pH value to get 0mV. This one can be calculated as follows : pH(E=0mV) = [pH7*E(pH4) pH4*E(pH7)]/(EpH4-EpH7)] = In previous example : pH(E=0mV) = [7.00*181 4.01*4] / [181-4] = (1267 16.04) / (177) = 7.067 5
Hamilton SUS pH electrodes During process calibration : Some processes may require pH adjustments of the electrode during process and without removing the electrode from the vessel. This procedure is called product calibration . The user takes a sample of medium (through sterile sample valve) and makes a reference pH measurement (in lab). In case the reference value differs from process value more than a user pre-defined tolerance, an adjustment is required. It consists of an offset adjustment, the slope remains unchanged. Hamilton SUS electrodes can be handled is this way, the procedure however must be supported by the controller / transmitter. 6
Thank you for your attention! Hamilton Bonaduz AG, Switzerland Hamilton Company, Reno / USA 7