Simeprevir Combination Therapy in Treatment-Naïve HCV Genotype 1 Patients: QUEST-1 Trial Results
The QUEST-1 trial evaluated the efficacy and safety of simeprevir in combination with peginterferon and ribavirin versus peginterferon and ribavirin alone in treatment-naïve patients with chronic HCV genotype 1 infection. The study showed a significantly higher proportion of patients achieving sustained virologic response at 12 weeks with the triple therapy compared to the dual therapy. Subgroup analysis demonstrated favorable outcomes for patients with HCV genotype 1a. Overall, the addition of simeprevir to standard therapy showed promising results in improving treatment outcomes for this patient population.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
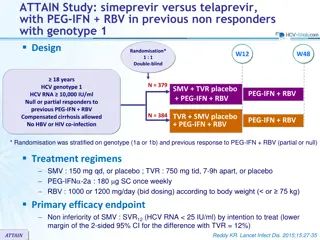
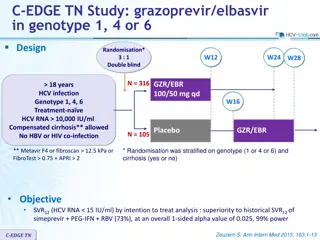
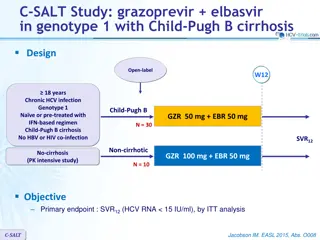
Phase 3 Treatment Na ve Simeprevir + PEG + RBV in Treatment-Na ve Genotype 1 QUEST-1 Trial Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + Ribavirin for Treatment-Nave HCV GT1 QUEST-1 Trial QUEST-1 Trial: Features Design: Randomized, double-blind, placebo-controlled, phase 3 trial with simeprevir + PEG + RBV versus PEG + RBV in treatment-na ve GT 1 Setting: Multicenter at 71 sites in 13 countries Entry Criteria - Treatment-na ve, chronic HCV monoinfection - HCV Genotype 1 (1a or 1b) Patient Characteristics - N = 394 - HCV Genotype: 1a (56%); 1b (44%) - IL28B Genotype: 71% non-CC - Age: median age 48 - Sex: 56% male - Race: 89% white, 8% black - Liver disease: F3 = 18%; F4 = 12% Primary end-points: Efficacy (SVR12) and safety Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Design 0 12 24 36 48 Week Response-Guided Therapy Patients with HCV RNA <25 IU/ml at week 4 and <15 IU/ml at week 12 completed treatment after 24 weeks. Randomized 2:1; stratified on IL28B and HCV1 subtype Simeprevir + PEG + RBV N = 264 PEG + RBV PEG + RBV Placebo + PEG + RBV N =130 PEG + RBV Drug Dosing Simeprevir: 150 mg once daily Peginterferon alfa-2a (PEG): 180 mcg/week Ribavirin (RBV) weight-based (in 2 divided doses): 1000 mg/day if < 75 kg or 1200 mg/day if 75kg Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Results QUEST-1: Proportion of Patients with SVR12 100 P < 0.0001 80 Patients (%) with SVR 12 80 60 50 40 20 210/264 65/130 0 Simeprevir + PEG + RBV PEG + RBV Abbreviations: SVR12 = sustained virologic response at 12 weeks; PEG = peginterferon; RBV = ribavirin Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Results SVR12 by HCV Genotype 1 Subtype Simeprevir + PEG + RBV PEG + RBV 100 90 Patients (%) with SVR 12 80 71 60 52 49 40 20 105/147 36/74 105/117 29/56 0 1a 1b HCV Genotype Abbreviations: PEG = Peginterferon; RBV = Ribavirin Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Results QUEST 2: SVR12 for HCV 1a by Baseline Q80K Status Simeprevir + PEG + RBV PEG + RBV 100 85 Patients (%) with SVR 12 80 60 53 52 44 40 20 31/60 16/30 73/86 19/43 0 1a (with baseline Q80K) 1a (without baseline Q80K) HCV Genotype Abbreviations: PEG = Peginterferon; RBV = Ribavirin Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Results SVR12 Response in Simeprevir Arm Based on Achievement of RGT Criteria Patients (%) who Met RGT Criteria SVR12 Based on Meeting RGT 100 Met RGT Criteria Did Not Meet RGT Criteria Unclassified 91 Patients (%) with SRV 12 80 60 11% 85% 4% 40 21 20 N = 264 203/224 6/28 0 Met RGT Did Not Meet RGT RGT= response-guided therapy: in simeprevir study arm, patients with HCV RNA<25 IU/ml at week 4 (undetectable or detectable) and <25 IU/ml at week 12 (undetectable) stopped treatment after 24 weeks Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Results QUEST 1: SVR12 by Host IL28B Genotype Simeprevir + PEG + RBV PEG + RBV 100 Patients (%) with SVR 12 94 80 78 76 60 65 40 42 20 24 72/77 29/37 114/50 32/76 24/37 4/17 0 CC CT TT Abbreviations: PEG = Peginterferon; RBV = Ribavirin Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Results QUEST 1: SVR12 by Liver Fibrosis (Metavir Score) Simeprevir + PEG + RBV PEG + RBV 100 Patients (%) with SVR 12 83 78 80 60 58 60 40 29 26 20 152/183 54/90 36/46 6/23 18/31 5/17 0 F0-F2 F3 F4 (Cirrhosis) Metavir Fibrosis Score Abbreviations: PEG = Peginterferon ; RBV = Ribavirin Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Results On-Treatment Failure or Relapse Simeprevir + PEG + RBV PEG + RBV 60 50 Patients (%) 40 34 30 21 20 9 9 10 24/264 44/130 21/234 18/84 0 On-Treatment Failure Relapse Stopping rules: (1) Stop simeprevir or placebo if HCV RNA>1000 at week 4; (2) Stop all therapy if HCV RNA < 2 log10 IU/mL reduction at week 12; (3) Stop all therapy if HCV RNA 25 IU/mL at week 24 or 36. On-treatment failure: Detectable HCV RNA at end of treatment. Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Results Emergent Protease Resistance in Patients who Failed to Achieve SVR12 Among simeprevir-treated patients who failed to achieve SVR12, emergent mutations in NS3 protease domain detected in 35 (92%) of 38 Genotype 1A: Most common mutation = R155K alone or in combination with mutations at codons 80 and/or 168 Genotype 1B: Most common mutation = D168V Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Adverse Effects Placebo + PEG + RBV (n=130) QUEST 1: Event Simeprevir + PEG + RBV (n=264) Discontinuation (due to adverse event) 3% 2% Grade 3 adverse event 25% 33% Grade 4 adverse event 3% 5% Fatigue 42% 41% Headache 33% 39% Pruritus 30% 20% Rash (any type) 34% 32% Anemia 20% 21% Photosensitivity condition 3% <1% Neutropenia 24% 18% Bilirubin increase 9% 5% Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
Simeprevir + PEG + RBV for Treatment-Nave HCV GT1 QUEST-1 Trial: Conclusions Interpretation: Simeprevir once daily with peginterferon alfa 2a and ribavirin shortens therapy in treatment-naive patients with HCV genotype 1 infection without worsening the adverse event profiles associated with peginterferon alfa 2a plus ribavirin. Source: Jacobson IM, et al. Lancet. 2014;384:403-13.
This slide deck is from the University of Washingtons Hepatitis C Online and Hepatitis Web Study projects. Hepatitis C Online www.hepatitisc.uw.edu Hepatitis Web Study http://depts.washington.edu/hepstudy/ Funded by a grant from the Centers for Disease Control and Prevention.