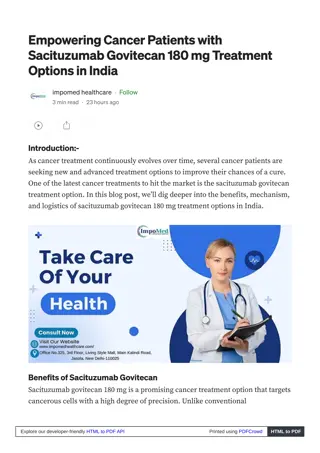
Sacituzumab Govitecan 180 mg Improving Quality of Life for Cancer Patients
Sacituzumab Govitecan 180 mg Injection significantly improves the quality of life for cancer patients by targeting the Trop-2 protein on tumor cells and delivering precise treatment. The availability of Sacituzumab Govitecan 180 mg in India ensures a
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
(+91) 8750275027 contact@impomedhealthcare.com C-70, SECOND FLOOR OFFICE -A, DDA SHEDS, OKHLA INDUSTRIAL AREA, PHASE- 1, NEW DELHI-110020. Home Who We Are What We Do Diseases Treatment FAQs Contact Us SACITUZUMAB GOVITECAN-HZIY Home | Cancer | Solid Tumors | SACITUZUMAB GOVITECAN-HZIY SACITUZUMAB GOVITECAN-HZIY Injection Description Composition Dosage Indications Contact Us SACITUZUMAB GOVITECAN 180 MG INJECTION TRODELVY (sacituzumab govitecan-hziy) is a Trop-2 directed antibody and topoisomerase inhibitor conjugate, composed of the following three components: the humanized monoclonal antibody, hRS7 IgG1 (also called sacituzumab), which binds to Trop-2 (the trophoblast cell-surface antigen-2); the drug SN-38, a topoisomerase inhibitor; a hydrolysable linker (called CL2A), which links the humanized monoclonal antibody to SN-38.... * TRODELVY (sacituzumab govitecan-hziy) for injection is a sterile, preservative-free, off-white to yellowish lyophilized powder for intravenous use in a 50 mL clear glass single-dose vial, with a rubber stopper and crimp-sealed with an aluminum flip-off cap. nWhere n~7.6 SN-38/Mab *Each single-dose vial of TRODELVY delivers 180 mg sacituzumab govitecan-hziy, 77.3 mg 2-(N-morpholino) ethane sulfonic acid (MES), 1.8 mg polysorbate 80 and 154 mg trehalose dihydrate. Reconstitution with 20 mL of 0.9% Sodium Chloride Injection, USP, results in a concentration of 10 mg/mL. C-70, SECOND FLOOR OFFICE -A, DDA SHEDS, OKHLA INDUSTRIAL AREA, PHASE- 1, NEW DELHI-110020. +91-9007090388 +91-8750275027 contact@impomedhealthcare.com rahman@impomedhealthcare.com mohan.singh@impomendhealthcare.com Quick Links Diseases Corporate O?ce Home Who We Are What We Do Treatment FAQs Contact Us Blog Cancer Neurology Endocrinology Miscellaneous C-70, SECOND FLOOR OFFICE -A, DDA SHEDS, OKHLA INDUSTRIAL AREA, PHASE- 1, NEW DELHI-110020. , +91-8750275027 +91-9007090388 Established in New Delhi, India in 2020, ImpoMed Healthcare has grown over the years from a new start up to one of the Most Preferred Company for Facilitation of the USFDA/European FDA Approved. contact@impomedhealthcare.com rahman@impomedhealthcare.com Imported Drugs-Only mohan.singh@impomendhealthcare.com Follow us on: Copyright 2024 ImpoMed