Reactions of Metals with Acids
In this educational material, we explore the reactions of metals with acids, their products, and the varying behaviors of different metals. The content covers the reaction equations, observations, and interesting facts like the use of aqua regia to dissolve gold and platinum. Dive into the world of chemistry as metals interact with different acid concentrations, showcasing unique chemical properties.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
What happens when Metals react with Acids?
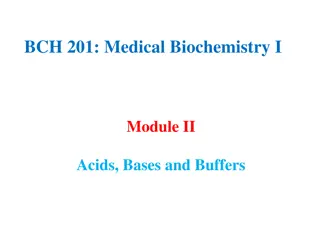
We have already learnt that metals react with acids to give a salt and hydrogen gas. Metal + Dilute acid Salt + Hydrogen But do all metals react in the same manner? Let us find out. Collect all the metal samples except sodium and potassium again. Mg, Zn, Cu, Fe, Al If the samples are tarnished, rub them clean with sand paper.
CAUTION: Do not take Sodium and Potassium as they react vigorously even with cold water. Put the samples separately in test tubes containing dilute Hydrochloric acid. Suspend thermometers in the test tubes, so that their bulbs are dipped in the acid. *Observe the rate of formation of bubbles carefully. *Which metals reacted vigorously with dilute hydrochloric acid? *With which metal did you record the highest temperature? Arrange the metals in the decreasing order of reactivity with dilute acids.
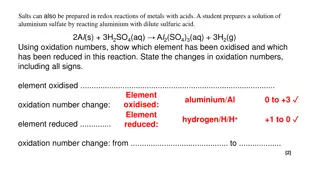
Write Magnesium, Aluminium, Zinc and Iron with dilute hydrochloric acid. Mg + 2HCl---------- MgCl2 +H2 2 Al + 6 HCl---------- 2AlCl3 + 3H2 Zn +2HCl---------- ZnCl2 + H2 2Fe +6HCl-------- 2FeCl3 + 3H2 equations for the reactions of *Copper does not react with dilute HCl.
Hydrogen gas is not evolved when a metal reacts with nitric acid. It is because HNO3 is a strong oxidising agent. It oxidises the H2 produced to water and itself gets reduced to any of the nitrogen oxides (N2O, NO, NO2). But Magnesium (Mg) and Manganese (Mn) react with very dilute HNO3 to evolve H2 gas.
if the solution is very dilute: Mg + 2HNO3-------- Mg(NO3)2 + H2 At moderate concentrations (and even with very dilute acid, this will happen to some extent): 3Mg +8HNO3-------- 3 Mg(NO3)2 + 2NO + 4H2O And with concentrated acid: Mg + 4HNO3-------- Mg(NO3)2 + 2NO2 + 2H2O
Do you know.. Aqua regia Aqua regia, (Latin for royalwater ) is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3:1. It can dissolve gold, even though neither of these acids can do so alone. Aqua regia is a highly corrosive, fuming liquid. It is one of the few reagents that is able to dissolve gold and platinum.
FOR MORE QUERIES: YOU CAN LOG IN TO WEBSITE www.scienceeasylearning.wordpress.com YOU CAN ALSO LIKE MY FB PAGE THAT IS: KKCHAUHAN https://www.facebook.com/kkchauhanvision/