Comparison of Aliphatic and Aromatic Carboxylic Acids in Chemical Properties
The article explores the differences between aliphatic and aromatic carboxylic acids, detailing their properties, common uses in food and cosmetics, as well as their reactions in ignition tests. It discusses the appearance changes and odor variations upon ignition, along with the distinctive color flames and residues produced. A comparison is made regarding the inflammability, luminosity, smoke production, and transformation characteristics of the various carboxylic acids. Additionally, it elucidates the specific odors emitted by different acids when subjected to heat.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
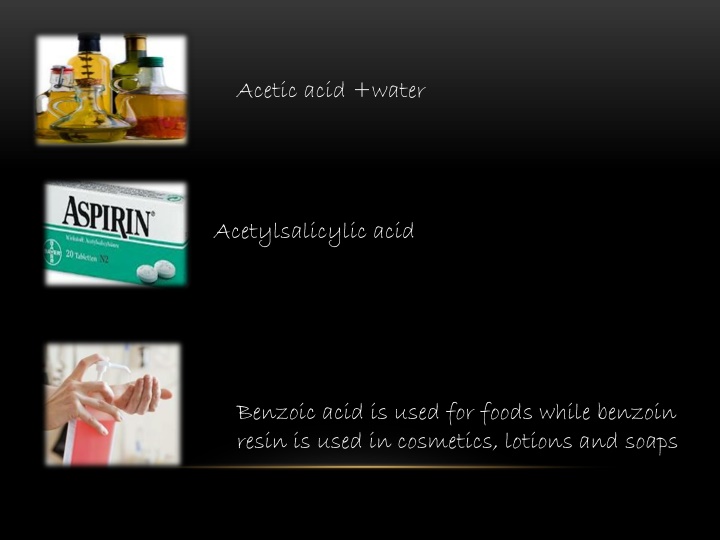
Acetic acid +water Acetylsalicylic acid Benzoic acid is used for foods while benzoin resin is used in cosmetics, lotions and soaps
Carboxylic acids Carboxylic acids Aliphatic carboxylic acids Aliphatic carboxylic acids http://upload.wikimedia.org/wikipedia/commons/thumb/1/17/Ameisens%C3%A4ure_Keilstrich.svg/120px-Ameisens%C3%A4ure_Keilstrich.svg.png Formic acid pungent Formic acid pungent true true liqid liqid mobily mobily http://upload.wikimedia.org/wikipedia/commons/thumb/0/08/Acetic-acid-2D-skeletal.svg/121px-Acetic-acid-2D-skeletal.svg.png Acetic acid colorless Acetic acid colorless vinegar vinegar http://upload.wikimedia.org/wikipedia/commons/thumb/0/05/Tartaric_acid.svg/200px-Tartaric_acid.svg.png Tartaric acid Tartaric acid solid crystalline solid crystalline http://upload.wikimedia.org/wikipedia/commons/thumb/c/c5/Zitronens%C3%A4ure_-_Citric_acid.svg/200px-Zitronens%C3%A4ure_-_Citric_acid.svg.png Citric acid yellowish white Citric acid yellowish white odorless odorless
Aromatic carboxylic acids Aromatic carboxylic acids http://upload.wikimedia.org/wikipedia/commons/thumb/0/0d/Benzoes%C3%A4ure.svg/110px-Benzoes%C3%A4ure.svg.png Benzoic acid Benzoic acid solid crystalline, solid crystalline, yellowish white odorless odorless yellowish white http://upload.wikimedia.org/wikipedia/commons/thumb/8/8e/Salicylic-acid-skeletal.svg/120px-Salicylic-acid-skeletal.svg.png Salicylic acid Salicylic acid http://upload.wikimedia.org/wikipedia/commons/thumb/c/c8/Phthalic-acid-2D-skeletal.png/150px-Phthalic-acid-2D-skeletal.png Phthalic Phthalic acid solid flaky , acid solid flaky ,yellowish white yellowish white odorless odorless
Ignition test Ignition test Aliphatic C.A: Aliphatic C.A: inflammable ,non luminous and no smoky inflammable ,non luminous and no smoky Aromatic C.A: Aromatic C.A: inflammable , luminous and smoky inflammable , luminous and smoky Appearance change: Appearance change: formic and acetic acid volatilization formic and acetic acid volatilization tartaric acid swelling up and tartaric acid swelling up and charring* citric acid melting and formation yellow resin* citric acid melting and formation yellow resin*blackening benzoic, salicylic, benzoic, salicylic, phthalic phthalic acid melting acid melting charring* blackening
Color flame: Color flame: No change with all No change with all Odor: Odor: Formic ,acetic acid : no change Formic ,acetic acid : no change Tartaric, citric acid: Tartaric, citric acid: burnt sugar odor Benzoic , Benzoic ,phthalic phthalic acid: irritant odor (suffocating). acid: irritant odor (suffocating). Salicylic acid: phenolic odor . Salicylic acid: phenolic odor . burnt sugar odor. . Residue: Residue: No residue with all except No residue with all except Tartaric acid : black residue. Tartaric acid : black residue. Citric acid: yellow stain. Citric acid: yellow stain.
Acid base test Acid base test Acidity test: Acidity test: Na Na2 2CO CO3 3 solution( solution(conc conc) + acid ) + acid warm warm effervescent effervescent Strong effervescent that means acid. Strong effervescent that means acid. Weak effervescent that means acidic. Weak effervescent that means acidic. Conc. Conc. Hcl when substance soluble in when substance soluble in 10 HcL HcL in same test tube in same test tube reprecipted Hcl test: test: 10% reprecipted again % NaOH NaOH that means acid .if add that means acid .if add again
acids acids Formic Formic Acetic Acetic tartaric tartaric citric citric benzoic benzoic phthalic phthalic salicylic salicylic H H2 2O O Miscible Miscible L. L.P P Acidity test Acidity test Conc. Conc. HCL HCL Change Change blue to red to red blue Change blue Change blue to red to red soluble soluble effervescent effervescent Reppt Reppt. . Insoluble in Insoluble in H H2 2O , O ,dil HCL but HCL but soluble in soluble in 10 10%NaOH %NaOH dil Not done Not done All strong acid All strong acid
Preliminary test Preliminary test Soda lime test: Soda lime test: Formic and acetic acid: not done for solid only. Tartaric and citric acid: burnt sugar odor on hot. Benzoic and phthalic acid: benzene odor on hot. Salicylic acid: phenolic odor on hot.
30 30%NaOH: %NaOH: The same result of soda lime FeCL FeCL3 3: : No reaction with all. Conc. H Conc. H2 2SO Formic and acetic acid Formic and acetic acid: effervescent due to CO2 evolution on cold. Tartaric acid Tartaric acid : effervescent and heavy charring on hot. Citric acid Citric acid: effervescent and yellow stain after long time it blackening. blackening. benzoic, benzoic, phthalic phthalic and salicylic acids and salicylic acids: no reaction SO4 4:
General test General test Neutra Neutral l FeCL FeCL3 3: : 1 1 ml ml of acids +2ml NH4OH heat until complete disapperance of ammonia odor then wait 2-3 min. to cooling then add 2-3 dps of neutral FeCL3 formic ,acetic acids: formic ,acetic acids: blood red Tartaric, citric acids: Tartaric, citric acids: lemon yellow Benzoic, Benzoic, phthalic phthalic acids: Salicylic acid: Salicylic acid: no need to use NH4OH just salicylic +H2O +neutralFeCL3 to give violet color. blood red color give brown if heat it. lemon yellow color. acids: buff ppt.
Differentiation tests Formic acid Turbidity or whit ppt. (Hg2CL2) Disappear violet color, form brown ppt. (manganus oxide Acetic acid - HgCL2: HgCL2+acid (wait 5 min) Alkaline Kmno4: acid + Na2CO3 sol. +KMNO4 - Esterfication : ethOH +acid + Conc. H2SO4 heat then pour it onto H2O Fruity or ripe apple odor -
Tartaric acid Eff. And charring Citric acid Eff. And yellow stain Discoloration of violet color anf form white ppt. Conc. H2SO4: (hot) Denges: acid+ H2O+ denges reagent boiling (without cooling) + KMNO4 - Benzoic acid Phthalic acid Unstable pink color Phthaline test: Phenol+ acid +Conc. H2SO4 heat then pour it onto 10% NaOH Flourescence; Acid + resercinol+ Conc. H2SO4 heat then pour it onto 10% NaOH - Reddish brown color give green flourescence if dilute it with water -
Salicylic acid Esterfication: methanol+ saliucylic acid+ Conc H2SO4heat then pour it onto 10 ml of H2O vicks odor of winter green oil due to methyl salicylate formation. Formaldehyde: Formaldehyde+ salicylic acid + Conc. H2SO4 crimson red color on the wall. Phthaline test: Salicylic acid + phthalic unhydride +Conc. H2SO4 heat then pour it onto 10 ml of 10% NaOH unstable pink color
IR location IR location COOH stretching band at 1710 cm-1