Potato Photosynthesis: Molecular Models and Chemical Changes
Explore the process of photosynthesis in potato plants through molecular models and chemical changes at different scales, from atomic-molecular to macroscopic. Learn how potato plants produce food through biosynthesis and cellular respiration, connecting the atomic-molecular scale to the macroscopic scale. Understand how atoms bond together in molecules and the role of chemical energy in these bonds. Through creating reactant molecules like carbon dioxide and water, delve into the intricate process of converting energy units of light into products.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
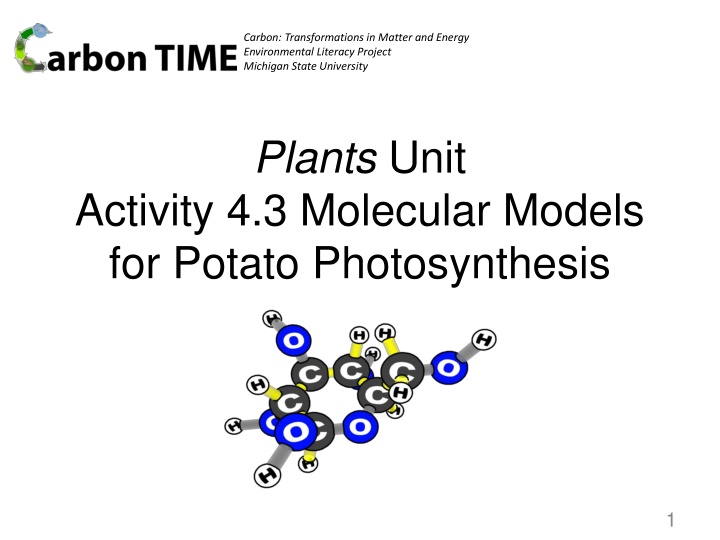
Carbon: Transformations in Matter and Energy Environmental Literacy Project Michigan State University Plants Unit Activity 4.3 Molecular Models for Potato Photosynthesis 1
You are here
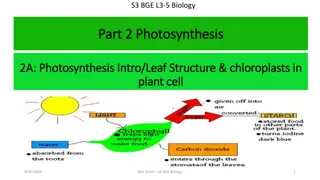
Connecting Questions about Processes at Different Scales Scale Unanswered Questions How does a potato plant make the food it needs to grow and function? Macroscopic Scale How do potato s leaf cells produce the food the potato needs to grow and function? Microscopic Scale What chemical change produces food in the potato s leaf cells? Atomic-Molecular Scale 3
How does a potato plant make the food it needs to grow and function? Materials for growth: Biosynthesis Food To Cells Energy: Cellular respiration 4
Connecting the Atomic-Molecular Scale to the Macroscopic Scale Chemical change 5
How atoms bond together in molecules Atoms in stable molecules always have a certain number of bonds to other atoms: Carbon: 4 bonds Oxygen: 2 bonds Hydrogen: 1 bond Oxygen atoms do NOT bond to other oxygen atoms if they can bond to carbon or hydrogen instead. Chemical energy is stored in bonds between atoms Some bonds (C-C and C-H) have high chemical energy Other bonds (C-O and O-H) have low chemical energy 6
Making the reactant molecules: carbon dioxide and water Complete Step 1 in Part B of your worksheet. 7
Photo of reactant molecules: CO2 (carbon dioxide) and H2O (water) Start by making the molecules of the reactants and energy units of light. Put them on the reactants side, then rearrange the atoms and energy units to show the products. Chemical change Carbon dioxide Water Products Reactants Remember: Atoms last forever (so you can rearrange atoms into new molecules, but can t add or subtract atoms). Energy lasts forever (so you can change forms of energy, but energy units can t appear or go away). 8
Important: When you are finished constructing the reactants, put all extra pieces away. 9
Record your results Record your results for your reactants. 10
Rearranging the Atoms to Make Product Molecules: Glucose and Oxygen Complete Step 2 in Part B of your worksheet. 11
Photo of product molecules: H6C12O6 (glucose) and O2 (oxygen) Start by making the molecules and energy units of the reactants and putting them on the reactants side, then rearrange the atoms and energy units to show the products. . Glucose Chemical change Oxygen Products Reactants Remember: Atoms last forever (so you can rearrange atoms into new molecules, but can t add or subtract atoms). Energy lasts forever (so you can change forms of energy, but energy units can t appear or go away). 12
Comparing photos of reactant and product molecules Compare the atoms and energy units on the reactant and products sides. . Glucose Chemical change Carbon dioxide Water Oxygen Products Reactants Remember: Atoms last forever (so you can rearrange atoms into new molecules, but can t add or subtract atoms). Energy lasts forever (so you can change forms of energy, but energy units can t appear or go away). 13
Record your results Record your results for your products. 14
What happens to atoms and energy in photosynthesis? Carbon Dioxide Glucose Reactants Chemical change Water Products Oxygen Light energy 15
What happens to carbon atoms in photosynthesis? Carbon Dioxide Glucose Reactants Chemical change Water Products Carbon atoms in carbon dioxide become part of glucose molecules. Oxygen Light energy 16
What happens to oxygen and hydrogen atoms in photosynthesis? Carbon Dioxide Glucose Reactants Chemical change Water Products Oxygen and hydrogen atoms become part of glucose and oxygen gas molecules. Oxygen Light energy 17
What happens to light energy in photosynthesis? Carbon Dioxide Glucose Reactants Chemical change Water Products Light energy is transformed into chemical energy. Oxygen Light energy 18
What happens to atoms and energy in photosynthesis? Carbon Dioxide Glucose Reactants Chemical change Water Products Atoms last forever! Energy lasts forever! Oxygen Light energy 19
Discuss with the class 1. Did the number and type of atoms stay the same at the beginning and end of the chemical change? ____ 2. Did the number of twist ties (representing energy) stay the same at the beginning and end of the chemical change? ____ 3. Why do the numbers of atoms and twist ties have to stay the same? 20
Writing a Chemical Equation Chemists use chemical equations to show how atoms of reactant molecules are rearranged to make product molecules Writing the equation in symbols: Chemists use an arrow to show how reactants change into products: [reactant molecule formulas] product molecule formulas] Saying it in words: Chemists read the arrow as yield or yields: [reactant molecule names] yield [product molecule names] Equations must be balanced: Atoms last forever, so reactant and product molecules must have the same number of each kind of atom Try it: can you write a balanced chemical equation to show the chemical change when animals move (use energy)? 21
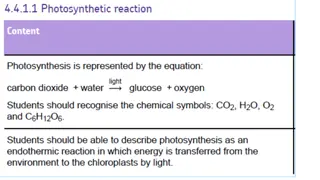
Chemical equation for photosynthesis 6 CO2 + 6 H2O C6H12O6 + 6O2 (in words: carbon dioxide reacts with water to yield glucose and oxygen) 22
Exit Ticket Conclusions Where do you think the reactants for photosynthesis come from? Predictions Where do you think photosynthesis occurs? 23