Overview of NHSN Healthcare Worker Influenza Vaccination Module
Influenza vaccination among healthcare personnel is crucial due to the potential spread of the virus and the high-risk patient population they interact with. Monitoring vaccination rates helps prevent outbreaks, severe illness, and supports national health goals. Reporting benefits include tracking trends, assessing program effectiveness, and meeting federal requirements. Expectations involve participation in reporting programs like the CMS IQR. Reporting begins this year on a voluntary basis and becomes mandatory in subsequent years. Updates in the vaccination module aim to capture summary data efficiently.
- Influenza vaccination
- Healthcare personnel
- Reporting requirements
- Vaccination rates
- National health goals
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
1 OVERVIEW OF THE NHSN HEALTHCARE WORKER INFLUENZA VACCINATION MODULE AND REPORTING REQUIREMENTS September 26, 2012
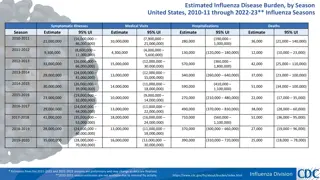
Why is influenza vaccination being measured among healthcare personnel? 2 Influenza can be spread by an ill person one day before to 7 days after onset of symptoms. Healthcare personnel (HCP) care for, or are in frequent contact with, patients with influenza or at high risk for complications of influenza. Vaccination can help prevent illness, outbreaks, and death, decrease severity of illness, and avoid losses in productivity. HCP flu vaccination rates are part of the U.S. s national health goals: Healthy People 2010: 60% Healthy People 2020: 90% Data systems can capture facility, state, and regional trends on the yearly uptake of the vaccine.
Benefits for the facility 3 Provide a record for flu vaccination and adverse reactions for HCP in the facility Monitor trends in vaccination and declination rates Assess efficacy of facility influenza vaccination programs Meet requirement for record-keeping for adult vaccine administration Comply with federal reporting requirements
What are the expectations for reporting? 4 Mandate of participation in the Centers for Medicare and Medicaid Services (CMS) Hospital Inpatient Quality Reporting (IQR) Program Applies to acute care hospitals Data entered into the National Healthcare Safety Network (NHSN) http://www.cdc.gov/nhsn/PDFs/HPS- manual/Operational-Guidance-HPS-Flu- Vaccination-Sum-Acute-Care.pdf No state mandate for reporting at this time
What is expected this year vs. subsequent years? 5 This flu season (2012-2013) Reporting begins January 1, 2013 (voluntary to report beginning Oct 1, 2012) and ends March 31, 2013 Data due to NHSN by May 15, 2013 CDC will share all in-plan HCP influenza vaccination summary data with CMS. A hospital-specific HCP flu vaccination percentage will be provided for each reporting hospital. Facility can edit data after May 15 but changes will not be sent to CMS. Changes after June 30th of an influenza season may not be used for national reporting by CDC for that season. Subsequent flu seasons (2013-2014 and on) Reporting mandatory from Oct 1 Mar 31 (entire reporting period) Mandatory reporting for long-term acute care hospitals begins with 2013-2014 season
Whats new about the current forms and module? 6 Vaccination Module of the Healthcare Personnel Safety Component in NHSN revised Sept 2012 Captures summary data, not individual-level data New protocol, forms, and surveys Healthcare Personnel Safety Monthly Reporting Plan Form Healthcare Personnel Influenza Vaccination Summary Form Seasonal Survey on Influenza Vaccination Programs for Healthcare Personnel (coming soon Jan 2013)
Getting started 7 Enroll facility in NHSN Activate Healthcare Personnel Safety Component Facility Administrator responsible On left toolbar, click Facility Add/Edit Components Check box indicating that the facility will be following the Healthcare Personnel Safety Component Define the primary contact for this component in Contact Data section of the page
Getting started (contd) 8 Make sure primary contact has administrative rights To create an NHSN user account: On left toolbar of NHSN, click Users Add Add name, e-mail address and save information Edit User Rights screen will pop up next. Check appropriate boxes to grant rights and click Save . If already an NHSN user On left toolbar of NHSN, click on Users Find Search for person or click Find to see all users Click on person s name and Edit to be able to change his/her rights. Check appropriate boxes and save the changes.
Getting started (contd) 9 Primary contact for HPS Component ensures staff who will be entering the vaccination data have user rights Primary contact (who has admin rights) can grant the rights New user will have to agree to NHSN rules of behavior, then apply for and install a digital certificate before being able to use the system
Notes about the reporting plan 11 Data must be in-plan in order to be shared with CMS. Once the Influenza Vaccination Survey box is checked on a monthly reporting plan, the NHSN system will auto-check that box on every monthly reporting plan throughout the NHSN-defined influenza season (July 1 June 30)
Who are healthcare personnel? 12 All employees on payroll Licensed independent practitioners (who are physicians, advanced practice nurses, and physician assistants affiliated with the hospital but not on payroll) Students, trainees, and volunteers Other contract personnel (not mandatory that you report on vaccination status of these staff) See Appendix A of HCP Vaccination Module: Influenza Vaccination Summary Protocol Must be aged 18 or older Must physically work in the facility for at least 30 days between October 1 and March 31 to be counted
Other notes about classification of HCP 13 Include all HCP who have worked at the facility for at least 30 working days regardless of clinical responsibility or patient contact. Working for any number of hours a day counts as one working day. Include both full-time and part-time persons. Count individuals not full-time equivalents. If someone works in two or more facilities, each facility should include that person in its denominator. HCP categories are mutually exclusive count each HCP only once.
Vaccination status categories 15 Vaccinated at healthcare facility Vaccinated outside healthcare facility (need written report or documentation) A signed statement or form, or an electronic form or e- mail from a healthcare worker (HCW) indicating when and where he/she received the influenza vaccine A note, receipt, vaccination card, etc. from the outside vaccinating entity stating that the HCW received the influenza vaccine at that location
Vaccination status categories (contd) 16 Medical contraindication Severe allergic reaction to eggs or other vaccine components History of Guillain-Barr Syndrome within 6 weeks after a previous flu vaccination HCP can provide written or verbal documentation of medical contraindications Declined vaccine (includes religious exemptions, other medical conditions) HCP can provide verbal or written declination of the vaccine Unknown vaccination status (or criteria not met for categories above)
Reporting 18 HCP influenza summary reporting consists of a single data entry screen per influenza season. NHSN defines a flu season as July 1 June 30. Data covering the entire reporting period (Oct 1 Mar 31) must be entered once into NHSN for each reporting year. The data can be entered on a monthly and/or quarterly basis, but only cumulative data should be entered. Note: any new data will overwritethe previous data. The system will auto-fill date last modified . Message confirming that data were saved will appear at the top of the screen. Remember, these are summary data and reflect the overall vaccination status of HCP in your facility for the entire flu season to date.
Analysis 19 Not covered in-depth during this webinar CDC training materials do have some screenshots and sample reports From left toolbar: Analysis Output Options HCW Vaccination Module Influenza CDC Defined Output Examples of analyses: Number of HCP Vaccinated, Number of HCP Working During the Required Time Period, Percentage of Vaccination Compliance, Percentage of Vaccination Non-Compliance
How will these data be publicly reported? 20 HCP influenza vaccination rates will not be publicly reported for the 2012-2013 season. Beginning with 2013-2014 season, facility-specific HCP influenza vaccination rates are projected to be posted on the Hospital Compare website.
Where can I get more information? 21 NHSN Vaccination Module page: http://www.cdc.gov/nhsn/hps_vacc.html Protocol: http://www.cdc.gov/nhsn/PDFs/HPS-manual/HPS- protocol-Vaccination-Mod-Influenza-Vaccination- Summary.pdf FAQs: http://www.cdc.gov/nhsn/faqs/FAQ-Influenza- Vaccination-Summary-Reporting.html Operational guidance for complying with CMS reporting requirements: http://www.cdc.gov/nhsn/PDFs/HPS- manual/Operational-Guidance-HPS-Flu-Vaccination-Sum- Acute-Care.pdf NHSN@cdc.gov include HPS Flu Summary in the subject line of the e-mail
NHSN training 22 http://www.cdc.gov/nhsn/Training/healthcare- personnel-safety/index.html Two slidesets one on overview of HPS Component, one on overview of vaccination module CDC webinars offered 10/3 (12-1PM), 10/11 (2-3PM). Registration is full; however, will be archived and available soon afterward: http://www2.cdc.gov/vaccines/ed/nhsn Covers data entry and analysis
Questions? 23 Andrea Alvarez Virginia Department of Health Healthcare-Associated Infections Program Coordinator Andrea.Alvarez@vdh.virginia.gov 804-864-8097