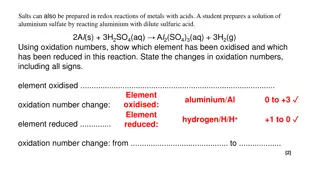
Overview of Furan: Preparation, Properties, and Reactions
Furan, a heterocyclic compound, is prepared by distillation of mucic acid or decarbonylation of furfural. It exhibits aromatic character, undergoing addition and substitution reactions like hydrogenation, Diels-Alder reaction, halogenation, nitration, sulphonation, and Friedel-Crafts reaction. Known for its diverse chemistry in both organic and physical aspects.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
FURAN Class: II B. Sc., Zoology Sub: Organic and Physical Chemistry Sub Code: 17UCHA41 Dr. S. SIVAKUMAR Assistant Professor PG Department of Chemistry Hajee Karutha Rowther Howdia College, Uthamapalayam - 625 533.
Preparation of Furan Preparation Furan is prepared by the dry distillation of mucic acid and heating the product (furoic acid).
Preparation of Furan Manufacture Furan is prepared on a large scale by the catalytic decarbonylation of furfural in steam in the presence of calcium oxide catalyst.
Properties of Furan Furan behaves both as a diene and as an aromatic compound. However, the aromatic character dominates. Thus, furan undergoes addition and substitution reactions.
Properties of Furan 1. Addition reactions i) Hydrogenation Furan is catalytically reduced to tetrahydrofuran (THF).
Properties of Furan ii) Diels Alder reaction Furan behaves as a diene and undergoes Diels Alder reaction with maleic anhydride.
Properties of Furan 2. Substitution reactions i) Halogenation 2 Bromo furan is obtained when furan is treated with bromine in dioxin at 00C.
Properties of Furan ii) Nitration Furan undergo nitration to form 2-Nitro furan
Properties of Furan iii) Sulphonation Furan undergo sulphonation with sulphur trioxide in pyridine at 700C to get Furan-2 sulphonic acid.
Properties of Furan iv) Friedel Crafts reaction Furan can be acetylated with acetic anhydride in the presence of BF3in ether solution at 00C.
Properties of Furan v) Formation of organolithium compounds Furan reacts with butyl lithium to form 2 furan lithium
Properties of Furan 3. Gattermann Koch reaction Furan when treated with a mixture of hydrogen cyanide and hydrogen chloride in the presence of anhydrous ZnCl2yields furfural.