In Vitro Culture of Ovule and Ovary for Haploid Plant Production
In vitro culture of unpollinated ovaries and ovules offers an alternative method for haploid plant production. This process involves isolating and culturing ovules in a chemically defined nutrient medium to understand zygote development and embryo maturation stages. Techniques for collecting, preparing, and culturing the ovules are outlined, with a focus on achieving successful haploid plant production from various plant species. The use of liquid medium, sucrose as a carbon source, and the addition of auxins for growth stimulation are highlighted in the procedure.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
INTRODUCTION Invitro culture of unpollinated ovaries and ovules represents an alternative for the production of haploid plant First successful report on the induction of gynogenic haploid was in barley by San Noeum in 1976 Haploid plants are obtained from ovary and ovule culture of rice, wheat, maize, sunflower, tobacco, poplar, mulberry etc
When liquid medium is employed the ovaries can be placed on filter paper raft Sucrose carbon source Maltose, lactose equally favourable Addition auxin greater stimulation of growth
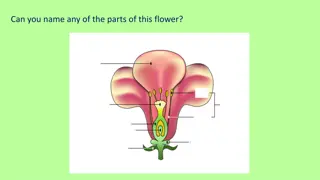
Culture of ovule ovules are aseptically isolated from the ovary and are grown aseptically on chemically defined nutrient medium under controlled conditions ovule is a mega sporangium covered by integument Ovules are attached with placenta inside the ovary by means of its funiculus
Ovules can be isolated and cultured in nutrient medium In vitro ovule culture helps to understand the factors that regulate the development of a zygote through organized stages to a mature embryo
PROCEDURE Collect the open flower (unfertilized ovules). If fertilized ovules are desired, collect the open flower where the anthers are dehisced and pollination has taken place. To ensure the fertilization, collect the flower after 48 hrs. of anther dehiscence.
PROCEDURE Remove sepals, petals, androecium etc. from the ovaries containing either fertilized or unfertilized ovules. Soak the ovaries in 6% NaOCl solution. Rinse the ovaries 3-4 times with sterile distilled water.
PROCEDURE Using sterile techniques, ovules are gently prodded with the help of spoon shaped spatula by breaking the funicles at its junction The spatula with ovules is gently lowered into the sterile solid or liquid medium as the culture vial is slanted about 45 .
PROCEDURE Damaged or undersized ovules are rejected when possible, during transfer. Incubate the ovule culture in either dark or light (16 hrs. 3,000 lux) at 25 .C
Advantages In a normal process these hybrids fail to develop due to early embryo abortion and premature abscission of fruits. Thus ovule culture can be used to rescue them This technique omits excision of the embryo by culturing the entire ovule.
Culture of ovary Ovary culture is a technique of culture of ovaries isolated either from pollinated or un- pollinated flowers Developed by Nitsch in 1951 Ovary is a ovule bearing region of a pistil Medium containing mineral salt and sucrose Vitamin B, IAA, coconut milk
For many species e.g. tomato, gherkin (Cucumis anguria) excised ovaries grow in culture and form the fruits that ripen and produce viable seeds provided the flowers have been fertilized two or more days before excision
Ovaries of un-pollinated flowers do not grow on simple nutrient medium Use of some synthetic auxins such as 2, 4-D, 2, 4, 5- T (2, 4-5- trichlorophenoxyacetic acid), NOA (2, Napthoxyacetic acid) in the nutrient medium induces the development of ovaries of un- pollinated flowers.
Procedure Collect the pollinated or un-pollinated flowers from a healthy plant. Wash them thoroughly with tap water, dip into 5% Teepol solution for 10 minutes and again wash to remove the trace of Teepol. Transfer the flowers to laminar air flow cabinet. Surface sterilizes the flowers by immersing in 5% sodium hypochlorite solution for 5-7 minutes.
Procedure Wash them with sterile distilled water. Transfer the flowers to a sterile petridish. Using a flamed forceps and a surgical scalpel, dissect out the calyx, petals, anther filaments etc. of the flower to isolate the ovary
Procedure Place the ovaries on agar solidified nutrient medium. Incubate the cultures at 25 C in a 16 hrs, daylight region provided by fluorescence tubes
Advantages useful to study the early development of embryo development, fruit development, different aspects of fruit physiology including respiration, maturation and disease effect of phytohormones on parthenocarpic fruit development can be studied from the culture of un-pollinated pistil.
factors affecting seed set after in vitro pollination Physiological state of explants The physiological state of the pistil at the time of excising the ovules or ovary influences the seed pollination. Wetting surface of the ovules or stigma may lead to poor pollen germination or bursting of the pollen tubes and poor seed set. set after in vitro
To improve the chances of success of in vitro pollination the level of incompatibility should be reduced The time of excising the ovules from pistil has a definite influences on seed set after in vitro pollination. Ovules excised 1-2 days after anthesis show a higher seed set.
Culture medium Nutrient medium on successful culture of ovules include Nitsch s mineral salts, white s vitamins and 5% sucrose. Several orchid ovules isolated from pollinated ovaries can grow successful on simple 10% sucrose solution and Zephyranthes require coconut milk or casamino acids.
Culture medium Kinetin promotes the initial growth of the embryo. 10mgL-1 IAA or 0.1mg kinetin improves the no. of seeds per ovule. Nitsch s medium is appropriate for in vitro culture of pollinated ovules of most species.
Culture medium Osmolarity of the culture medium also affects the development of excised ovule. Sucrose anywhere in the range 4-10%.
Storage condition Usually the first step of this process occurs at room temperature and without special lighting. The ovary cultures are maintained at 22-26 C and other suitable conditions favouring embryogenesis.
Genotype The response of in vitro ovaries in relation to the seed set depends on the species. Pollen grains of crucifers are difficult to germinate in cultures and a modified technique is required to obtain germinable seed.
Applications Overcoming self-incompatibility: Petunia axilaris and Petunia hybrida are self- incompatible species. Germination of pollen is good on self- pollinated pistils but a barrier exists in the zone of the ovary as a result, the pollen tube cannot fertilize the ovule. The barrier of these taxa can be overcome by in vitro pollination
Applications Production of stress-tolerant plant : Maize plants tolerant to beat stress have been produced through in vitro pollination. Additionally these plants exhibited increased vigour and grain yield.
Applications Development of young hybrid embryo: Development of young hybrid embryos can be achieved in extremely widely crosses through in vitro pollination. The efficiency of this technique needs much improvement.