Exploring the Fascinating World of Molecular Boranes and Their Applications
Molecular boranes, with a rich history dating back a century, have diverse applications ranging from rocket fuel to cancer therapy. This article delves into the identification of the first borane, advancements in metal borohydrides, and the synthesis of borane anions. The discussion extends to the compounds B10H10²⁻ and B12H12²⁻, along with their derivatives, and explores the emerging applications of closo-boranes in solid-state ion conductivity and medicine. Additionally, insights into the synthesis and structures of silver boranes are highlighted.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
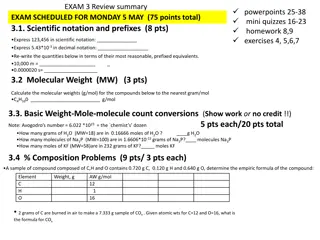
Introduction Molecular boranes have been known for 100 years! -First borane correctly identified (B4H10) in 1912 by A. Stock Molecular boranes have a rich history in rocket fuel applications -Boranes (B-H) 70,000 kJ/kg -Alkanes (C-H) 42,000 kJ/kg Metal borohydrides first reported in 1940 by Schlesinger, Brown & Burg: Al(BH4)3, Be(BH4)2, LiBH4 F. M. Hawthorne synthesized the B12H122- anion in 1960 Nobel Prize in Chemistry 1976: W. N. Lipscomb for his studies on the structure of boranes illuminating problems of chemical bonding Nobel Prize in Chemistry 1979: H. C. Brown for boron compounds as reducing agents in organic chemistry 2 B. Hansen, brsh@chem.au.dk, MH2016
Borane Anions Closo BnHn 5 Arachno BnHn+6 Nido BnHn+4 6 7 10.1016/j.ccr.2015.12.003 8 9 10 11 12 3 B. Hansen, brsh@chem.au.dk, MH2016
Metal Borane Synthesis Hansen et al. Coord Chem Rev, 2016, 10.1016/j.ccr.2015.12.003 4 B. Hansen, brsh@chem.au.dk, MH2016
B10H102- and B12H122- compounds and derivatives NH4+ Table available here: Hansen et al. Coord Chem Rev, 2016 10.1016/j.ccr.2015.12.003 Often hydrates or solvates! 5 B. Hansen, brsh@chem.au.dk, MH2016
Closo-borane applications Ion conductors Medicine (BNCT) Cancer Therapy PCCP, 2013,15, 15825-15828 NH3/H2 storage Carborane-containing amino acids Li2B12H12 7NH3 J. Mater. Chem., 2010, 20, 2743-2745 Nano-Car Texas, USA Polymers/Ceramics Luminescence Polym. Chem., 2011, 2, 2122 Org. Lett., 2006, 8 (8), 1713 1716 J. Mater. Chem. A, 2015, 3, 22853 6 B. Hansen, brsh@chem.au.dk, MH2016
Solid-State Ion Conductivity Hansen et al. Coord Chem Rev, 2016, 10.1016/j.ccr.2015.12.003 7 B. Hansen, brsh@chem.au.dk, MH2016
Silver Boranes: Synthesis Hydroxides, metals, nitrates or oxides can also be used instead of carbonates = 0.9938 Muetterties, Chemistry of Boranes VIII, salts and acids of B10H102-and B12H122-,1964 Paskevicius et al. Nature Energy, 2016, submitted 8 B. Hansen, brsh@chem.au.dk, MH2016
Silver Boranes: Structures -Ag2B12H12 Pa-3 -Ag2B12H12 Fm-3m 12.5% 12.5% 50% 12.5% 12.5% Paddlewheel mechanism B12H122- anion highly dynamic Ag+ in partially occupied sites with much closer jumps Only certain Ag-sites are permitted for particular B12H122- orientations (Ag-H distance) Boron, Silver Paskevicius et al. Nature Energy, 2016, submitted 9 B. Hansen, brsh@chem.au.dk, MH2016
Silver Boranes: Ion Conductivity Au Sample Au Electrochemical Impedance Spectroscopy Mark Paskevicius Silver boranes, worth their weight in gold? Tuesday, Room 3 Paskevicius et al. Nature Energy, 2016, submitted 10 B. Hansen, brsh@chem.au.dk, MH2016
NH3 storage in closo-boranes Or indirect H storage MB12H12 M = Li2, Na2, Ca Flask placed in cooling bath Aim is to study structures and decomposition mechanism = 1.5406 Hansen et al. J. Phys. Chem. C, 2016, to be submitted 11 B. Hansen, brsh@chem.au.dk, MH2016
NH3 storage in closo-boranes In situ SR-PXD of ammoniated Na2B12H12 Na2B12H12 2NH3 Trigonal, P-3m1 (no. 164) a = 7.1672(1) , c = 7.1574(2) , HT-Na2B12H12 LT-Na2B12H12 Na2B12H12 xNH3 Na2B12H12 4NH3 Monoclinic, P21/n (no. 14) a = 8.6875(2) , b = 9.4168(3) , c = 9.9096(3) , = 98.3296(18) = 0.7750 Boron, Sodium, Nitrogen, Hydrogen Hansen et al. J. Phys. Chem. C, 2016, to be submitted 12 B. Hansen, brsh@chem.au.dk, MH2016
NH3 storage in closo-boranes Na2B12H12 Compound NH3content (wt%) H content from NH3(wt%) Total H content (wt%) Li2B12H12 7NH3 43.36 7.70 12.10 Na2B12H12 4NH3 CaB12H12 6NH3 26.62 4.73 9.45 CaB12H12 35.97 6.39 10.64 Thermal and chemical stability advantageous No formation of BN Reversible = 1.5406 Hansen et al. J. Phys. Chem. C, 2016, to be submitted 13 B. Hansen, brsh@chem.au.dk, MH2016
NH3 storage in closo-boranes Reversible NH3 storage Thermal and chemical stability advantageous No formation of BN Reversible Hansen et al. J. Phys. Chem. C, 2016, to be submitted 14 B. Hansen, brsh@chem.au.dk, MH2016
Conclusions Revisit old methods to make new materials o Extensive research foundation in the literature on metal boranes New materials lead to new and exciting properties o Ag2B10H10 and Ag2B12H12 are excellent ion conductors and have interesting structures o Ammine metal closo-boranes can be useful for reversible NH3/H2 storage or as DeNOX catalysts There are large number of metal boranes to be explored for a broad number of applications GO AND EXPLORE NOW! 15 B. Hansen, brsh@chem.au.dk, MH2016
Acknowledgements Funding: Torben R. Jensen Mark Paskevicius Bo Richter Mathias J rgensen Anders S. Jacobsen Hai-wen Li Etsuo Akiba Nikolay Tumanov Yaroslav Filinchuk Antonio Santoru Claudio Pistidda Martin Dornheim Mark Paskevicius HAPPY BIRTHDAY ( Yesterday) Thank you for your attention! 16 B. Hansen, brsh@chem.au.dk, MH2016