Exploring the Fascinating World of Molecular Boranes and Their Applications
Molecular boranes, with a rich history dating back a century, have diverse applications ranging from rocket fuel to cancer therapy. This article delves into the identification of the first borane, advancements in metal borohydrides, and the synthesis of borane anions. The discussion extends to the c
0 views • 17 slides
Electrolyte and Metabolic Disturbances in the Critically Ill
Dr. Srilekha Ammapalli discusses the cations (sodium, potassium, calcium, magnesium) and anions (chloride, bicarbonate, phosphate, lactate) involved in electrolyte and metabolic disturbances in critically ill patients. The physiology, causes of abnormalities, diagnosis, evaluation, and management of
2 views • 104 slides
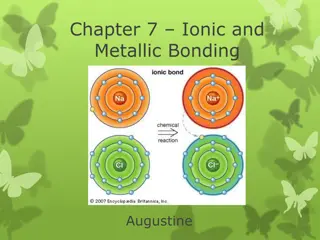
Understanding Ionic and Metallic Bonding: Valence Electrons, Octet Rule, and Ion Formation
Explore the essential concepts of ionic and metallic bonding, focusing on valence electrons, electron dot structures, the octet rule, cations, anions, and ion formation. Discover how atoms achieve stability through electron transfer, and learn to write electron configurations for various ions.
9 views • 52 slides
Understanding Ionic and Metallic Bonding in Chemistry
Explore the concepts of ionic and metallic bonding in chemistry through discussions on valence electrons, electron dot structures, the octet rule, cations, anions, and more. Dive into the world of ions and electron configurations to understand how atoms achieve stability through the gain or loss of
3 views • 62 slides
Microplastic Occurrence in South Korean Groundwater by Well Depth and Hydrogeology
The study conducted by Kangwon National University in South Korea analyzed microplastic occurrence in groundwater from wells of varying depths and hydrogeological settings. Samples were collected from the National Groundwater Monitoring Network pipes in Gapyeong and Chuncheon. Water type analysis re
7 views • 6 slides
Understanding Ionic and Metallic Bonding in Chemistry
Explore the concepts of ions, electron dot structures, the octet rule, cations, and anions in Chapter 7. Learn how elements achieve stability through electron configurations, and practice writing electron dot structures and naming ions. Understand the differences between cations and anions and how t
1 views • 52 slides
Understanding Borane Cluster Structures and Styx Rule
Boranes are cluster compounds of boron and hydrogen with unique structural arrangements. The Skeletal Electron Count and Styx Rule help in determining the bonding features and types of bonds present in borane compounds. Various borane structures such as Closo, Nido, and Arachno exhibit different bon
1 views • 11 slides
Factors Affecting Enzyme Activity and Catalysis
Enzyme activity is influenced by various factors such as enzyme concentration, temperature, pH, substrate concentration, inhibitors, activators, and physical agents. The rate of enzyme-catalyzed reactions is directly proportional to enzyme concentration, and temperature plays a significant role with
0 views • 23 slides
Understanding Crystal Field Theory in Chemistry
Crystal Field Theory (CFT) explains how electron orbital degeneracies, particularly d or f orbitals, are affected by a static electric field generated by neighboring anions. In CFT, the metal ion is considered positive while ligands are negative charges, leading to attractive and repulsive forces af
0 views • 13 slides
Theories of Electrolytic Dissociation and Ionization in Physical Chemistry
The theories of electrolytic dissociation by Adil Hamid and Arrhenius explain how electrolytes dissociate into ions in solution, leading to electrical conductivity. This process involves the migration of cations and anions towards opposite electrodes, affecting the conductivity of the electrolyte. T
1 views • 51 slides
Understanding Ionization of Carboxylic Acids
Carboxylic acids, as proton donors, can undergo ionization to form ions in chemical reactions based on the Brønsted-Lowry theory. Ionization involves the complete loss or gain of electrons, leading to the formation of cations (positively charged ions) and anions (negatively charged ions). Through e
1 views • 13 slides
Role of Major Physiological Anions in the Human Body
Physiological anions such as chlorides, sulphates, bicarbonate, phosphates, and electrolytes play essential roles in maintaining various functions within the body. Chloride ions help in osmotic balance, charge balance, and acid-base balance. Sulphates are important for detoxification mechanisms and
0 views • 11 slides
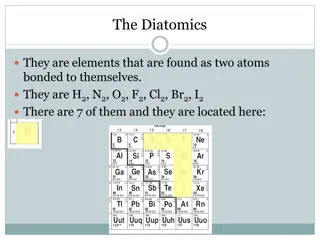
Exploring Periodic Trends and Diatomic Elements
Dive into the world of periodic trends, atomic size variations, ionization energy, electronegativity, and diatomic elements like H2, N2, O2, F2, Cl2, Br2, and I2. Discover how atomic structure influences these properties and learn about the fascinating characteristics of cations, anions, and shieldi
0 views • 12 slides
Understanding Ions, Ionic Bonds, and Ionic Compounds
Ions are charged particles formed by gaining or losing electrons, leading to the formation of ionic bonds between positively charged cations and negatively charged anions. The octet rule guides electron configurations, and the periodic table helps predict ion formation. Ionic bonding involves electr
0 views • 13 slides
Understanding Basis Set Generation in Computational Chemistry
Detailed explanation and control over defining basis set functions, species labels, number of shells, basis set generation procedures, and solving the Schrödinger equation for ion generation. Explore schemes for generating multiple basis sets and the impact of extra charge on orbital localization i
0 views • 17 slides
Understanding Ionic Compound Formulation and Nomenclature
Ionic compounds consist of cations and anions combined in simple ratios to balance charges. The cross technique helps determine the correct ratio of ions. Reduction of ratios may be necessary in certain cases. Formulation and naming examples are provided to illustrate these concepts.
1 views • 12 slides
Essential Information on Naming Compounds, Cations, and Anions
Learn about the essential elements, symbols, and polyatomic ions you need to know for naming compounds correctly. Understand rules for naming compounds when elements combine, including diatomic molecules, halogens, oxygen, and sulfur. Explore polyatomic ions with different charges and their names to
0 views • 12 slides
Understanding Precipitation Reactions in Analytical Chemistry
Precipitation reactions play a crucial role in analytical chemistry, where cations and anions combine to form insoluble solids called precipitates. By following solubility rules, scientists can predict these reactions, aiding in identifying ions present in solutions. Properties, formation, and equil
0 views • 17 slides
Factors Affecting Acid Strength: Atoms, Size, Hybridization, and Electronegativity
The strength of acids is influenced by various factors such as the atom on which the negative charge of the acid's conjugate base rests, atom size, hybridization, and electronegativity. The stability of negative charges on atoms, atom size allowing charge delocalization, preferred orbital types for
0 views • 23 slides
Understanding Calcium Homeostasis and Its Importance in the Body
Calcium homeostasis plays a crucial role in various physiological functions, including bone integrity, blood clotting, enzymatic regulation, hormonal secretion, neurotransmitter release, nerve excitability, and muscle contraction. Bones act as a major calcium reservoir, with blood containing 50% ion
0 views • 17 slides
Understanding Groundwater Quality Assessment
Groundwater quality is vital for various purposes such as drinking, industrial, and irrigation water. Assessing the quality involves measuring physical, chemical, and biological constituents, including inorganic elements like bicarbonate, sulfate, chloride, and more. Major cations and anions play a
0 views • 22 slides
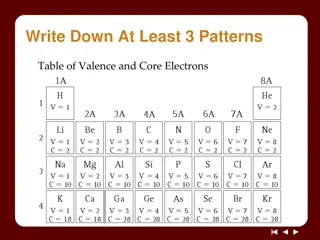
Understanding Ionic Bonds: Valence Electrons and Ion Formation
Exploring the concepts of valence and core electrons, Noble Gas Envy Ions, and the formation of ions through electron transfer between metal and nonmetal atoms. Learn about cations and anions, ion charges based on the periodic table, and the relationship between ion charge and valence electrons.
0 views • 13 slides