Understanding Molecular Shapes in Chemistry: A Practical Approach
Explore the fascinating world of molecular structures and properties by delving into the concept of molecular shape using formaldehyde (CH2O) as a model. Understand how the number of electron domains and bonding affect the overall shape of molecules, with practical activities to enhance learning.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Living By Chemistry SECOND EDITION Unit 2: SMELLS Molecular Structure and Properties
Lesson 38: Let s Build It Molecular Shape
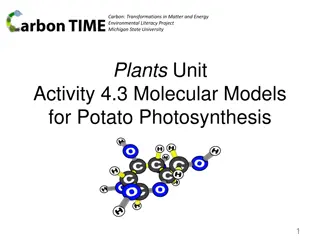
ChemCatalyst 1. What is the Lewis dot structure of formaldehyde, CH2O? Draw formaldehyde s structural formula. How many electron domains do you think this molecule has? Explain your reasoning. 2. 3.
Key Question How can you predict the shape of a molecule?
You will be able to: predict and explain molecular shape, including in molecules with multiple bonds
Prepare for the Activity Work in groups of four. Using the gumdrop, marshmallow, and toothpick kits, build a model of formaldehyde, CH2O.
Discussion Notes Double or triple bonding changes the number of electron domains around an atom, affecting the overall shape of a molecule. Trigonal planar shape: A flat triangular shape found in small molecules with three electron domains surrounding the central atom.
Discussion Notes (cont.) Linear shape: A geometric shape found in small molecules with two electron domains surrounding the central atom. The number of electron domains is more important in determining the structure of a molecule than is the number of atoms.
Discussion Notes (cont.) The more atoms in a molecule, the more combinations of shapes you might see together.
Wrap Up How can you predict the shape of a molecule? Drawing the Lewis dot structure of a molecule allows us to predict its three dimensional shape. The presence of double or triple bonds changes the number of electron domains around an atom, which in turn affects the overall shape of the molecule. The shape of large molecules is determined by the smaller shapes around individual atoms.
Check-In What is the shape of this molecule? H2S