Dose Error Reduction Software
Significance of Dose Error Reduction Software (DERS) in preventing medication errors. Learn how DERS creates individualized drug datasets and sets limits for dosing parameters. Discover real-life examples and the impact of DERS on patient safety.
Download Presentation

Please find below an Image/Link to download the presentation.
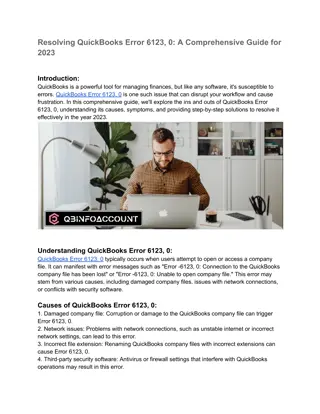
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Everything you need to know about Dose Error Reduction Software in 20 minutes or less Jeanie Misko Medicines Information Senior Pharmacist, FSH Breigh Ridley Team Lead Pharmacist, Critical Care, FSH
Objectives Defining Dose Error Reduction Software (DERS) Setting up a DERS library Maintaining a DERS library Resources and stakeholder engagement
What is DERS? Software to develop an individualised dataset of: Drugs Fluids Blood products Limits added to the variables of each drug: Dose Rate/duration Concentration
How DERS can Prevent Medication Errors Added layer of safety for patient and clinician Limits tailored to institution s guidelines Soft limits: can be overridden Hard limits: absolute min/max, no overrides Alerts outside limits prompt user to reconsider values entered Supportive clinical information at bedside
DERS Example Benzylpenicillin charted as 3500mg q4h, prepared & entered into DERS pump: 2400mg Benzylpenicillin - Dosing Limits 600mg 3200 mg 3500 mg BENZYLPENICILLIN DOSE NOT PERMITTED DOSE LIMIT 3200 MG
Without DERS Benzylpenicillin charted as 3500mg q4h, prepared & entered into pump : No alarms, no safety net Unusual dose may not be queried Patient receives wrong dose Potential adverse effects
FSH Near Miss Events 2016-2017 Near miss event = defined as 10 times or more over the hard maximum dose limit Top Near Miss Drugs Number of Near Miss Events Midazolam 30 Iron polymaltose 29 Heparin Noradrenaline 28 25 Insulin Actrapid 15
Resources to Build a DERS Library Pharmacy Medical Nursing IT Biomedical engineering Vendor Space! Establishment and maintenance
Timelines IV Guidelines Training Data entry Clinical review / testing Sign off Technical e.g. Wi-Fi
Lessons learnt Have your guidelines in place before you start developing your dataset Wi-Fi is strongly recommended Define roles and responsibilities early Consider ongoing FTE requirements Governance Documentation Consider the need for future vendor support Find your clinical champions
Can you use the FSH library? It depends Not a plug in and play! May not fit your institution s guidelines/needs Software compatibility Not for ongoing maintenance W.A. Health: share on request Private sites: negotiation at executive level
What about state-wide standardisation? It s possible! Need state based guidelines first Create momentum and drive for standardisation Potential for different levels of DERS libraries: E.g. Tertiary, secondary and rural sites
Clinical Review of the Paediatric and Neonatal Smart Infusion Pump Profiles Jeanie Misko1,Breigh Ridley1 1 Pharmacy Department, Fiona Stanley Hospital, Murdoch WA
Background DERS was introduced to FSH in October 2014 2016 average monthly DERS compliance Profile Adult profiles 86.1% Paediatric profiles 65.5% Neonates profiles 69.9%
Aims To create a working group to undertake a clinical review and update of paediatric and neonatal DERS profiles and To measure the impact of this on compliance and satisfaction.
Methods Identification and release of 14 members to participate in the multidisciplinary working group which met seven times throughout February 2017 8 x nursing, 4 x pharmacy, 2 x vendor, 0 x medical Electronic staff satisfaction survey for three weeks pre and post review Compliance with DERS measured using vendor supplied CQI software
Results recommended changes Type of Change Paediatric General Paediatric Critical Care Neonates Total (%) Name change Dose change Concentration change Infusion duration change Therapy change Weight banding change Clinical advisory change Delivery method change Addition of new drug Removal of drug Total 5 6 2 13 (5.5%) 76 (32.3%) 45 (19.1%) 54 (23.0%) 15 (6.4%) 6 (2.6%) 10 (4.3%) 10 (4.3%) 5 (2.1%) 1 (0.4%) 39 18 31 10 12 25 24 23 3 0 1 0 1 8 0 0 4 0 2 1 0 1 6 7 1 5 0 122 31 82 235
DERS compliance Aug 2016 Aug 2017 100 90 80 Percent compliance 70 60 50 40 30 20 review 10 0 Paediatric General Paediatric Critical Care Neonates Hospital Wide
DERS compliance Aug 2016 Aug 2017 DERS compliance % Aug 2016 Jan 2017 DERS compliance % March 2017 Aug 2017 Profile P-value Paediatric General 75.7 87.1 0.038 Paediatric Critical Care 61.9 82.9 <0.01 Neonates 76.5 96.7 <0.01 Hospital Wide 80.1 89.0 0.082
Staff Satisfaction 100 90 80 70 60 Percent 50 pre-review (n=63) 40 post-review (n=35) 30 20 10 0 staff satisfaction with DERS DERS meets clinical needs (drugs) DERS meets clinical needs (fluids) Most (65.3%) clinicians felt the changes had a positive impact on patient and clinician safety
Conclusion Review of paediatric and neonatal DERS profiles has resulted in Increased awareness of pharmacy s role in managing DERS alignment with guidelines improved clinician satisfaction improved DERS compliance Wide engagement and availability of stakeholders is crucial
Questions b FSH.PharmacyGuardrails@health.wa.gov.au

 undefined
undefined