Evaporation in the Food Industry
Evaporation, a key process in the food industry, involves removing excess water from raw materials or processed foods to enhance preservation and reduce costs. It requires considerations such as heat transfer, temperature control, and prevention of overheating. Evaporation differs from dehydration and drying, producing a concentrated liquid that can be further processed, like in the production of powdered products. The non-volatile components present impact the distinction between evaporation and distillation, with water being a common solvent in food processing. Despite benefits like mass reduction and preservation, concentrated products are more prone to chemical deterioration.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
EVAPORATION Dr. Sevim KAYA 1
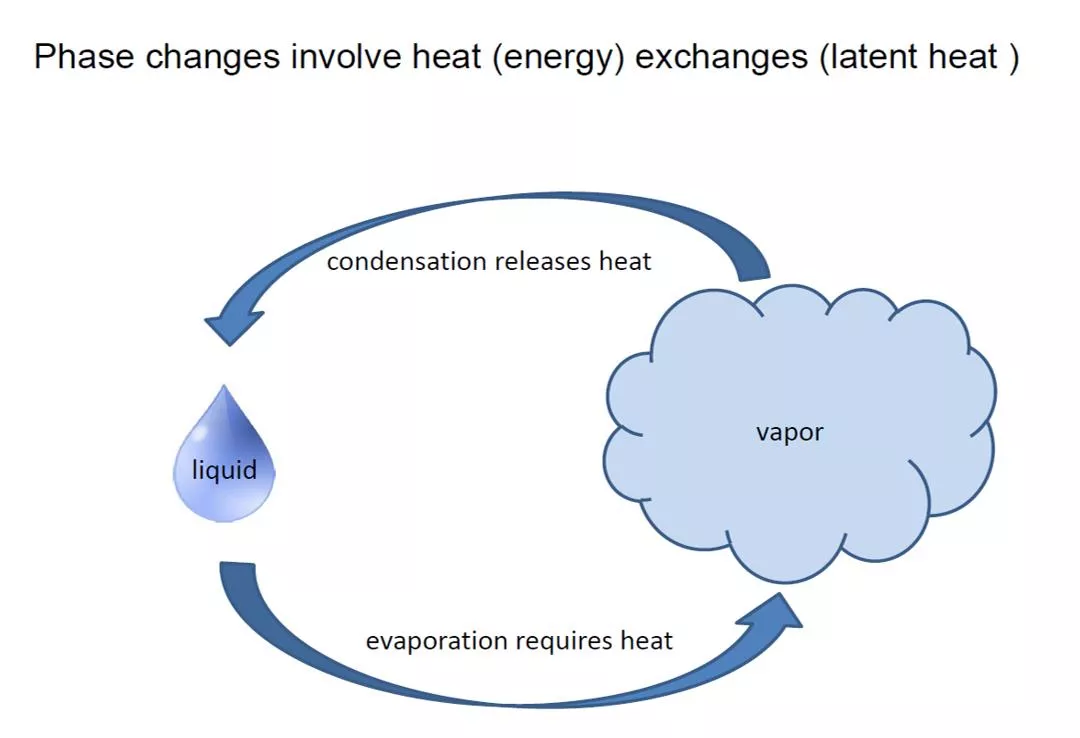
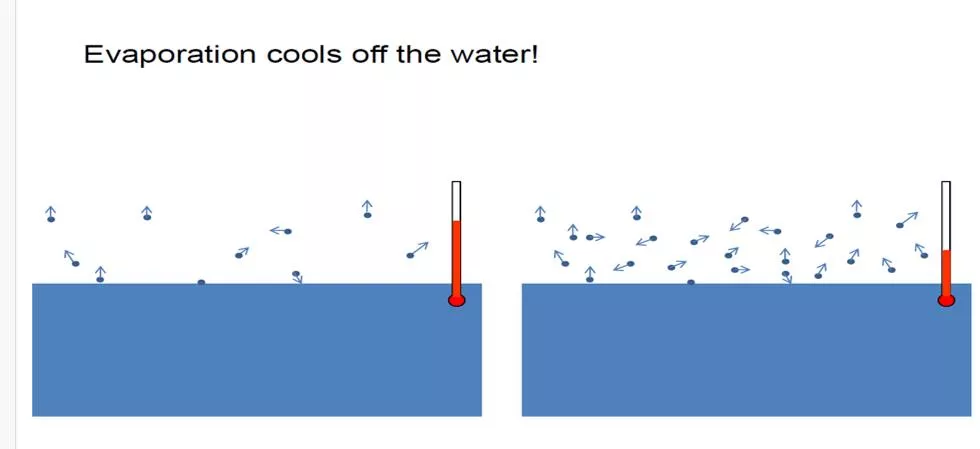
EVAPORATION Frequently in the food industry a raw material or a processed food contains more water than is required in the final product. When the foodstuff is a liquid, the easiest method of removing the water, in general, is to apply heat to evaporate it. Evaporation is thus a process that is often used by a food engineer. The basic factors that affect the rate of evaporation are the: rate at which heat can be transferred to the liquid, quantity of heat required to evaporate each kg of water, maximum allowable temperature of the liquid, pressure at which the evaporation takes place, changes that may occur in the foodstuff during the course of the evaporation process. 4
Considered as a piece of process plant, the evaporator has two principal functions, to exchange heat & to separate the vapor that is formed from the liquid. Important practical considerations in evaporators: maximum allowable temperature, which may be substantially below 100oC. promotion of circulation of the liquid across the heat transfer surfaces, to attain reasonably high heat transfer coefficients and to prevent any local overheating, viscosity of the fluid which will often increase substantially as the concentration of the dissolved materials increases (evaporating a portion of the solvent concentrates the solute into a more-viscous liquid product), tendency to foam which makes separation of liquid and vapor difficult. 5
Evaporation differs from dehydration and drying in that the product of evaporation is a concentrated liquid, not a solid. Evaporation can be used as the initial step in producing a dried product if the liquid concentrate then undergoes a drying process such as spray drying. The combination of evaporation and spray drying is often used to make powdered products, such as powdered milk. This combination of processes is economically attractive because high-efficiency evaporation is significantly less costly than drying and other methods of removing water. Evaporation also produces a higher concentration of solids than other methods of concentration. Evaporation may be carried out as a batch or continuous process. 6
The non-volatile nature of the components other than the solvent makes the difference between evaporation and distillation. In the case of foods, the volatile solvent is, almost always, water . The main objectives of evaporation in the food industry are the following: Mass and volume reduction, resulting in reduced cost of packaging, transportation and storage Preservation, by virtue of the reduced water activity. It should be noted, however, that the rate of chemical deterioration increases with concentration. Thus, concentrated juices, although more resistant than their single-strength form to microbial spoilage, tend to undergo browning more readily. (Refer to the effect of water activity on the rate of various types of food deterioration, discussed) Preparation to subsequent treatment such as crystallization (sugar, citric acid), precipitation (pectin, other gums), coagulation (cheese, yogurt), forming (candy), dehydration (milk, whey, coffee solubles) Building a desired consistency (jams and jellies, tomato concentrates, ketchup). 7
Evaporation is one of the most large-scale operations in the food industry. Plants with evaporation capacities of up to a hundred tons of water per hour are not uncommon. Made of special types of stainless steel, constructed according to high sanitary standards and equipped with sophisticated automatic control systems, evaporators for the food industry are relatively expensive. The most important running cost item in evaporation is energy. However, energy consumption per unit production can be reduced considerably through the use of multi-effect evaporation or vapor recompression or both. Since such measures also result in increased capital investment while reducing energy cost, their application is subject to optimization with respect to total cost. 8
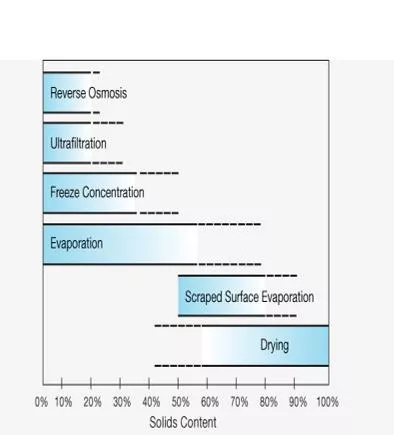
In the food industry, the issue of thermal damage to product quality deserves particular attention. Evaporation under reduced pressure and correspondingly lower boiling temperature is widely practiced. However, lower temperature means higher viscosity of the boiling liquid, slower heat transfer and therefore longer retention of the product in the evaporator. Thus, the selection of evaporation type and of operating conditions is also subject to optimization with respect to product quality. One of the problems in the evaporative concentration of fruit juices is the loss of volatile aroma components. Evaporators may be equipped with essence recovery systems for entrapping and concentrating the aroma, which is then added back to the Concentrate. Evaporation is not the only way to concentrate liquid foods. Alternative concentration processes are reverse osmosis, osmotic water transfer and freeze concentration, discussed in other parts of the book. 9
FACTORSEFFECTINGEVAPORATION: Concentration in the liquid: Liquid feed to an evaporator is relatively dilute. - So its viscosity is low, and heat-transfer coefficient high. As evaporation proceeds, the solution becomes concentrated. So viscosity increases and heat-transfer coefficient drops. Density and the boiling point of solution also increase. Solubility: As solution is heated, concentration of the solute in the solution increases. In case the solubility limit of the solute in solution is exceeded, then crystals may form. Solubility of the solute therefore determines the maximum concentration of the solute in the product stream. In most cases, the solubility of the solute increases with temperature. This means when a hot concentrated solution from an evaporator is cooled to room temperature, crystallization may occur. 10
Temperature sensitivity of materials: Foods are damaged when heated to moderate temperatures for relatively short times. So special techniques are employed to reduce temperature of the liquid and time of heating during evaporation Foaming and frothing: Solutions like organic compounds tend to foam and froth during vaporization. The foam is carried away along with vapor leaving the evaporator. Entrainment losses occur. 11
Pressure and temperature: The boiling point of the solution is related to the pressure of the system. The higher the operating pressure of the evaporator, the higher the temperature at boiling. Also, as the concentration of the dissolved material in solution increases by evaporation, the temperature of boiling may rise (a phenomenon known as boiling point rise/elevation). To keep the temperatures low in heat-sensitive materials, it is often necessary to operate under atmospheric pressure (that is, under vacuum). 12
Scale deposition: Some solutions deposit solid materials (called scale) on the heating surfaces. The result is that the overall heat-transfer coefficient (U) may drastically decrease, leading to shut down of the evaporators for cleaning purposes. Materials of construction: Evaporators are made of some kind of steel. However many solutions attack ferrous metals and are contaminated by them. Copper, nickel, stainless steels can also be used. 13
All evaporators are comprised of two sections: a heating section (called a steam chest) and a vapor/liquid separation section. These sections can be located within a single vessel (body), or the heating section may be external to the vessel that houses the vapor/liquid separation section. 14
SNGLE EFFECT EVAPORATORS Let F be the mass flow rate of feed. L be the mass flow rate of product. V be the mass flow rate of vapor. xF is the mass fraction of solute in the feed. xL is the mass fraction of solute in the product. and yV is the mass fraction of solute in the vapor. Total mass input = total mass output i.e. F= V + L Here we have neglected the steam as the amount of steam entering the steam chest is equal to the amount of condensate leaving the steam chest. Solute balance: FxF= L xL+ VyV If no solute is transferred into vapor, then yV = 0, that means FxF = L xL 15
ENERGY BALANCE: hF is the specific enthalpy of the feed. hV is the specific enthalpy of the vapor. hL is the specific enthalpy of the product. HS and hS are the specific enthalpy of saturated vapor (steam) and saturated liquid (condensate) respectively. P is the pressure in vapor space. TF is the feed temperature. T is the boiling point of the feed/solution corresponding to the pressure in vapor space. TS is the saturation temperature of the steam. CP is the specific heat of the feed. 16
Data provided: F = 9072 kg/h xF = 1 wt % = 0.01 kg solute / kg feed TF = 38C xL = 1.5 wt % = 0.015 kg solute / kg liquid product P = 101.325 kPa (1.0 atm abs) PS = 143.3 kPa U = 1704 W/m2 .K T1 = saturated temperature at P (= 101.325 kPa) = 100 C, TS = saturated temperature at 143.3 kPa = 383 C. Cp= 4.184 kJ/kg K 18
EFFECTSOFPROCESSINGVARIABLESON EVAPORATOROPERATION: Effect of feed temperature: The inlet temperature of the feed has a large effect on the evaporator operation. When feed is not at its boiling point, steam is needed first to heat the feed to its boiling post and then to evaporate it. Preheating the feed can reduce the size of evaporator heat transfer area 20
EFFECTOFPRESSURE: Pressure in the evaporator sets the boiling point of the solution (T1 ). Steam pressure determines the steam temperature (Ts ) Since q = U A (TS T1 ), larger values of (TS T1 ) will help reduce the heat-transfer area needed and hence the cost of evaporator. Vacuum can be maintained in the solution side using a vacuum pump. For example, if the pressure in Example 1 is reduced to 41.4 kPa, boiling point of water reduces to 349.9 K and that would increase the (TS T1 ) from 10 K to 33.3 K. A large decrease in heat-transfer area would be obtained. 21
EFFECTOFSTEAMPRESSURE: High pressure provides high Ts values, and hence TS T1 will increase. High pressure steam is however more costly. Therefore, overall economic balances must be considered to determine the optimum steam pressure. 22

 undefined
undefined