Chemical Pathways and Reactions
Delve into various chemical reactions and pathways, including cyclo compounds formation, diol synthesis from ethene, ozonolysis products, and industrial routes for ethanol synthesis. Uncover the diverse reagents and conditions leading to specific product formations in organic chemistry.
Uploaded on Mar 09, 2025 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
The Scrambled Full Monty Help meh !
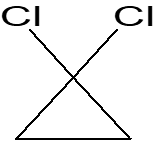
Provide examples of three different pathways leading to cyclo compounds Zn(Cu) Simmons-Smith insertion ethene + CH2I2 ------ a) OH- Cl Cl b) ethene +CHCl3-------- dichlorocarbene insertion light c) ethene + CH2N2 ----------- + N2 Diazomethane insertion Cl2 /Wet CCl4 ?????? H O Cl
Scrambled Exercise : (CONTINUED) HCl in acetic acid w/HOOH ?????? Cl B2H6 neat or in ether H H2O2 OH OH- ?????? All anti-mark, 90% yield 3 min,Brown 2 step Zn/H+ O3 neat H + ozonolysis O O H
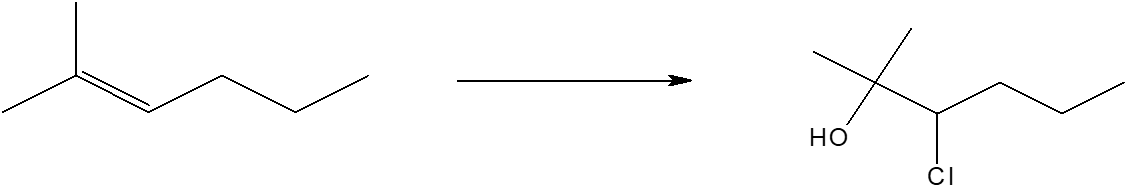
Scrambled Exercise 2: (CONTINUED) CHCl3/OH- Cl Cl ????? dichlorocarbene insertion CH2I2 Zn(Cu) ????? Simmons-Smith insertion Rxn name ??? HCl in acetic acid, no HOOH ????? Cl
Exercise 2: (CONTINUED) There are two alternative routes to make anti- versions of 1,2-dihydroxyethane (diols) from ethene. What are they ? Formic acid/peroxide ethene wet CCl4 OH- H+ ethene +Br2 b) OH a) H O CCl4/HOOH light Cl ????? Cl Cl Cl
Scrambled Exercise 2: (CONTINUED) There are two different choices of reagents leading to syn- versions of 1,2-dihydroxyethane from ethene. What are they ? a) KMnO4 (cold) H O OH b) OsO4 in ether NaBH4 in KOH H Hg(OAc)2 OH THF ????? What is the industrial route for converting ethene to ethanol? Conc H2SO4 H2O Ethene ------------ ethyl sulfonate---- ethanol + H2SO4
Scrambled Exercise 2: (CONTINUED) What alkene originally was present if the final product of ozonolysis is: O O H2 at 1 atm 25oC over Pt, Pd or Ni ??????
One more .polymerization patterns HO-OH/light a) n b) ??? HO-OH/light n ???
One more .polymerization patterns Predict the polymers formed by reacting: a) n HO-OH/light ???