Basics of Mammalian Cell Culture
Mammalian cell culture involves the removal of cells from an organism for growth in a controlled environment. Primary cell cultures can be sub-cultured, leading to the generation of cell lines. Sub-culturing involves transferring cells to fresh growth media for further growth. Different methods and equipment are required for maintaining cell cultures based on their requirements. Understanding the basics of cell culture is essential for successful research.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
INTRODUCTION TO MAMMALIAN CELL CULTURE Lecturer: Assist. Prof. Dr. Mirza Suljagi Assistant: Jasmin Sutkovic March, 16th 2018
Chapter 1 Introduction Textbook: Introduction to Mammalian Cell Culture (D. Ler, U. Glamo lija, M. Suljagi )
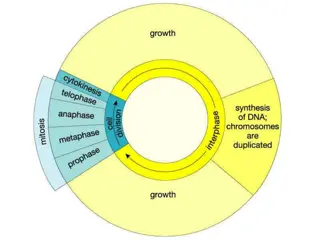
Introduction Cell Culture removal of cells from an organism and their subsequent growth in a favorable, artificially controlled environment Primary Cell Culture removal from tissue Can be sub-cultured Cell line due to proliferation Finite limited life span Immortal due to transformation (Continous cell line)
Primary Cell Culture Contains heterogenous cell population Sub-culturing leads to cell line generation Different purification methods Tissue sample: Dissection, Rinsing, Mechanical Dissagregation (Enz. Digestion) in trypsin Different separation techniques: Density gradient separation MACS FACS Figure Cell line
Sub-culturing cells are harvested, diluted in a fresh growth medium and replaced in a new culture flask to promote further growth Essential for viable state PRIMARY CUTURE Sub-culturing CELL LINE (SEC.CULTURE) Cell line transfer of cell from one to another culture vessel Providing fresh nutrients and growing space Figure Primary Cell Culture
Types of cell lines (on the basis of life span of culture): 1. Finite cell lines - limited life span - limited number of cell generations (40-60 doublings) - slow growth rate - doubling time 24-96 h 2. Continuous cell lines - transformed under laboratory conditions - grow in a monolayer or in suspension - rapid growth rate - doubling time 12-24 h Cell strain positive selection of cells either from primary culture or cell line by cell cloning Limited division potential, lose ability to proliferate (Hayflick limit)
CHAPTER 2 BASICS OF CELL CULTURE
Cell Culture Requirements and Equipment Differ depending on a cell culture (e.g. Cancer & GMO cells) Microbiologically free Equipment common to most cell culture labs: 1. Essential Equipment - cell culture hood, humid CO2 incubator, centrifuge, refrigerator (2-8 C) and freezer (-20 C) 2. Automated Cell Counter - inverted microscope, pipettor, micropipettes, cultureware 3. Outside culture environment - liquid nitrogen container, sterilizer (autoclave), waterbath
Work Surfaces Bench tops, wallsm flooring smooth and resistant to temperature and cracking (for liquid nitorgen) Major requirement to maintain aseptic work area Use cell culture hood (for aseptic conditions)
Basic Equipment 1. Clean Benches horizontal or vertical laminar flow - provide product protection - dust-free assembly of sterile equipment - never used for handling of cell cultures 2. Cell Culture Hood laminar flow cabinet or tissue culture hood - HEPA filter
Figure Laminar Flow Hood Figure Cell Culture Hood
Types of Cell Culture Hood Class I significant protection levels to lab personnel - no culture protection from contamination Class II designed for work involving BSL-1,2,3 materials - provide aseptic environment - for handling potentially hazardous materials (viruses, toxic/carcinogenic reagents, etc.) Class III highest level of protection - working with human pathogens and BSL-4 material
3. Incubators for growth conditions (temperature, CO2, humidity) Mammalian cell cultures at 37 C, 5-10% CO2 Large, forced air circulation, have temperature control 0.2 C, made of stainless steel Mammalian cells kept at humid CO2 incubators Humid CO2 incubators incubation of cells in sterile flasks Dry incubators water dish controles humidity
4. Centrifuge for maintanance of most cell lines and cyropreservation Balance the tubes Centrifuge rate 80-150 xg Higher forces may promote cell damage Tubes 15-50 ml Storage Storage areas for liquids (media, antibiotics) and consumables ( disposable pipettes, gloves, media bottles) Follow the instructions
5. Refrigerators and Freezers - for small cell culture lab (domestic refrigerators) Storing at -5 to -20 C Ultra-deep freezer - -80 C storing 6. Plastic-ware for Cell Culture Includes Petri-dishes, multi-well plates (6, 12, 24, 48, 96 wells per plate) Screwcap flasks classified acc. to surface areas (T-25, T-75, T-175, T-225 cm2) Provide hydrophilic surface to facilitate attachment of anchorage dependent cells
7. Inverted Microscope used to visualize cells in culture Easy to operate Useful for observing living cells or organisms at the bottom of a dish Provide information about cell morphology and cell state Cell culture can be viewed in flasks, dishes, multi-well plates Liquid Nitrogen cellular maintenance for prolonged period
Biosafety Main aim prevent injury, protect property, avoid harm to individuals and environment Common hazards: accidental punctures with syringe needles, spills, splashes onto skin and mucous membrane, inhalation, ingestion Strict adherence to standard microbiological practices and techniques Type of risks: a) Low risk non-human cont. cell lines (well characterized) b) Medium risk poorly characterized cell lines (mammalian) c) High risk primary cells derived from human/primate tissue
Biohazards Biological substances that represent a threat to human health Medical waste, toxins, viruses, m.o. Most common biohazards presented by cell culture human pathogenic viruses Cell products, biomolecules with modulations Figure Symbol of biohazard materials
Biosafety levels Biosafety in Microbiological and Biomedical Laboratories by NIH Describe safety equipment, microbiological practices 1. BSL-1 Basic level of protection Common to most labs For agents that are not known to cause disease to humans (Bacillus subtilis, Canine hepatitis, E.coli)
2. BSL-2 For moderate-risk agents Cause mild human disease (by ingestion) Work with lentiviral vectors (Zika, Hepatitis A, B, C, HIV, Salmonella) 3. BSL-3 For agents with known potential for aerosol transmission Agents may cause lethal infection (for which vaccines exist) West Nile Virus, Typhus, TBC, Plasmodium falciparum
4. BSL-4 For agents that pose high individual risk of life threatening disease No treatment Involves special equipment Contains UV light, showers, vacuum room, showers, autonomous detection system
Disinfection and Waste Disposal To minimize risk of contamination of cell culture Personal protective equipment should be used Main disinfectant: a) Hypochlorite (Sodium hypochlorite) - for general purpose - cannot be used on metal surfaces - should be made fresh daily - active against viruses b) Alcohol (Ethanol, Isopropanol) - effective against bacteria, viruses (only ethanol)
c) Aldehyde (Formaldehyde) - irritants, use should be limited due to sensitization problems - Used only in well ventilated areas Different waste forms (different treatments) Tissue culture waste Contaminated pipettes Solid waste
Aseptic Manipulations Keep the cells free from m.o. contamination Wear basic protective equipment Aseptic manipulation barrier between m.o and environment Elements of aseptic technique a) Sterile Work Area - use gloves, lab coat, cell culture hood - disinfect surrounding areas before and after use - Bunsen burner not recomended
b) Personal Hygiene - Wash hands to remove adherent m.o that would contaminate cell cultures - Keep places clean - Avoid talking, sneezing, coughing - One pipette cannot be used between two different bottles of media
Cell Culture Contamination Contaminants: a) Chemical media, sera, detergents, water, endotoxin impurities b) Biological bacteria, yeast, viruses, etc. Bacteria contamination is easily detected (pH drop, cloudly appearance, thin films on a surface) Yeast little pH change until the contamination is heavy - appear as individual ovoid or spherical particles Mycoplasmas Difficult to detect until they achieve extremely high densities - decrease proliferation rate, not cell death - fluorescent staining
Molds grow as multicellular filaments - appear as thin filaments or dense clumps of spores Viruses take over cell machinery to reproduce - hard to detect - detected by ELISA, PCR, electron microscopy