Analysis of BBDR Modeling for Human NPC Dose Linearity
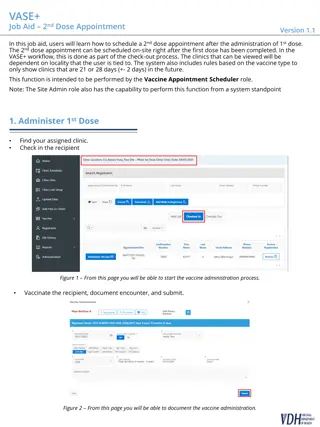
The BBDR modeling for human NPC dose linearity is examined through various studies and updates from 2003 to 2024. Key aspects include formaldehyde BBDR, regulatory acceptance/rejection, EPA/IRIS concerns, and the 2023 BBDR model update addressing main issues. The 2023 BBDR model presents two versions of nasal tumor response, suggesting no significant cancer risk below 0.3 ppm. The 2024 TSCA assessment is compared, highlighting inconsistencies and disregards of updated models and data.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
BBDR Modeling: The Data Do Not Support Low BBDR Modeling: The Data Do Not Support Low Dose Linearity for Human NPC Dose Linearity for Human NPC Rory B. Conolly, Sc.D. Presentation to USEPA SACC May 20, 2024 1
BBDR: Dosimetry + Model of Action BBDR: Dosimetry + Model of Action Dosimetry Mode of action/AOP
2003 1980s 1970s
Formaldehyde BBDR Formaldehyde BBDR Original publications Conolly et al. (2003) Rat BBDR. Toxicol. Sci. 75, 432-447 Conolly et al. (2004) Human BBDR. Toxicol. Sci. 82, 279-296 Updated model Conolly et al. (2023). Rat BBDR. Toxicol. Sci. 193, 1-17
Regulatory acceptance/rejection Regulatory acceptance/rejection The 2003, 2004 BBDR modeling was well received internationally MAK, 2002; WHO, 2002; Liteplo and Meek, 2003 EPA/IRIS stated that model uncertainties were too large for use in risk assessment Crump et al., 2008; Subramaniam et al., 2007; Subramaniam et al. 2008; USEPA 2010; USEPA 2022 2022 Draft IRIS assessment relied on these analyses.
2023 BBDR update: Main EPA/IRIS concerns 2023 BBDR update: Main EPA/IRIS concerns are addressed are addressed (we agreed with some, but not all) (we agreed with some, but not all) 1. Confidence in formaldehyde airflow and flux CFD model 2. Formaldehyde DNA adduct modeling 3. Uncertainty and variability in the rat cell labeling index data 4. Exact solution of the 2-stage clonal growth model 5. Assumption that rat nasal SCC are rapidly fatal 6. Use of historical control data 7. Uncertainties in division and death rates of initiated cells
2023 BBDR Model 2023 BBDR Model Two versions of nasal tumor response: 1. Cytotoxicity-driven cell division 2. Cytotoxicity plus mutagenic DNA adducts No significant cancer risk below 0.3 ppm in either case. Human version of the revised model expected to show similar behavior.
2024 TSCA Assessment 2024 TSCA Assessment Relies on 2022 draft IRIS assessment Ignores BBDR update published in March 2023 Human NPC arise by same mode of action as the rat nasal tumors Low dose linearity inconsistent with extensive mechanistic database Dose-response modeling from 1970s CFD dosimetry modeling is a centerpiece of the BBDR model but ignored by EPA Plenty of data to let the data inform the shape of the curve
Summary Summary Only BBDR modeling describes all the main determinants of the shape of the dose-response curve. EPA/IRIS critiques of the 2003/2004 BBDR modeling have been addressed in the 2023 update of the rat BBDR model Because the BBDR model describes the main determinants of the shape of the dose-response curve, refinement of the model improves the quality of the dose-response predictions and reduces uncertainty.
End End 10