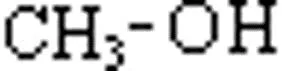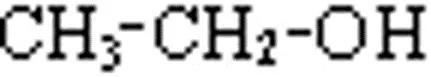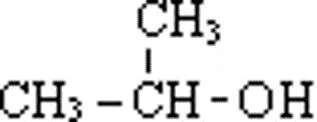Alcohols: Classification and Nomenclature
Alcohols are categorized into aliphatic and aromatic types based on the structure of the hydroxyl group. Aliphatic alcohols have the OH group attached to an aliphatic carbon chain, while aromatic alcohols have it in the side chain of an aromatic hydrocarbon. Further classification includes monohydric, dihydric, trihydric, and polyhydric alcohols based on the number of hydroxyl groups. Nomenclature follows the alkyl alcohol system for naming, with specific rules for indicating substituent positions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
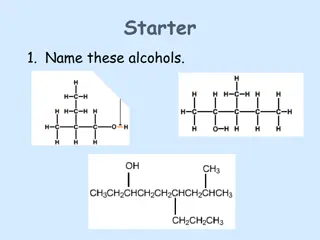
Classification of alcohols. All alcohols, principle, can be divided into two broad categories i. . aliphatic alcohols and aromatic alcohols. 1. Aliphatic alcohols. Alcohols in which the hydroxyl group is linked an aliphatic carbon chain are called aliphatic alcohols. For example, Methyl alcohol Ethyl alcohol Isopropyl alcohol Methanol Ethanol 2-Propanol
2. Aromatic alcohols. Alcohols in which the hydroxyl group is present in the side chain of an aromatic hydrocarbon are called aromatic For example. phenylmethanol 2-phenylethanol (benzyl alcohol) ( -phenylethyl alcohol) Alcohols are further classified as monohydric, dihydric, trihydric and lyhydric according as their molecules contain one, two, three, or many hydroxyl groups respectively. For m l , Ethyl alcohol 1,2-Ethanediol 1,2,3-propanetriol (Monohydric) (Dihydric) (Trihydric)
2. Aromatic alcohols. Alcohols in which the hydroxyl group is present in the side chain of an aromatic hydrocarbon are called aromatic For example. phenylmethanol 2-phenylethanol (benzyl alcohol) ( -phenylethyl alcohol) Alcohols are further classified as monohydric, dihydric, trihydric and lyhydric according as their molecules contain one, two, three, or many hydroxyl groups respectively. For m l , Ethyl alcohol 1,2-Ethanediol 1,2,3-propanetriol (Monohydric) (Dihydric) (Trihydric)
I. he alkyl alcohol system. In this system of common nomenclature, the name of an alcohol is derived by combining the name of the alkyl group with the word alcohol. The names are mitten as two words. n-butyl alcohol isobutyl alcohol tret-butyl alcohol II. In this common system, the position of an additional substituent is indicated by use of the Greek alphabet rather than by numbers. -chloroethyl alcohol -bromobutyl alcohol
Any simple radical that has common name may be used in the alkyl alcohol system, with one important exception. The grouping 6 5 - has the special name phenyl, but the compound C6H5OH is phenol, not phenyl alcohol. phenol Substituted phenols are named as derivatives of the parent compound phenol. The reason for this difference is historical and arose from the fact that phenol and its derivatives have many chemical properties that are very different from those of alkyl alcohols. However, phenyl substituted alkyl alcohols are normal alcohols and often have common names. Examples are: phenylmethanol 2-phenylethanol ( -phenylethyl alcohol (benzyl alcohol)
III. The carbinol system. In this system, the simplest alcohol, 3, is called carbinol. More complex alcohols are named as alkyl substituted carbinols. The names are written as one word. butylmethylcarbinol triethylcarbinol phenilcarbinol The number of carbons attached to the carbinol carbon distinguishes primary, secondary, and tertiary carbinols. As in the case of the alkyl halides, this classification is useful because the different types of alcohols show important differences in reactivity under given conditions. The carbinol system of nomenclature has been falling into disuse in recent years. However, it is found extensively in the older organic chemical literature.
Polyhydroxy alcohols: An alcohol in which two hydroxyl groups are present is named as diol, one containing three hydroxyl groups is named as triol, and so on. In these names for diols, triols, and so forth, the final of the parent alkane name is retained for pronunciation reasons. 1,2-Ethanediol 1,2-propanediol 1,2,3-propanetriol
Classification of monohydric alcohols Monohydroxy alcohols are hydrocarbon derivatives which contain only one group OH connected with sp -hybridizated carbon atom. The general formula of monohydroxy alcohols is: The names of monohydroxy alcohols are the names of the same hydrocarbons with added prefix ol.
Classification of monohydric alcohols. As already mentioned, alcohols containing one group per molecule are called monohydric alcohols. These are further classified as primary (1'), secondary (2'), and tertiary (3') according as the group is attached to primary, secondary and tertiary carbon atoms respectively. For example: Primary alcohol Secondary alcohol Tertiary alcohol Ethanol Isopropyl alcohol 2-Methylpropanane-2-ol