Alcohol Oxidation in Organic Chemistry
Explore the oxidation of alcohols in organic chemistry through a series of experiments involving primary, secondary, and tertiary alcohols. Learn how different alcohols react and identify the products of oxidation reactions. Understand the distinctions between aldehydes, ketones, and the classification of alcohols based on their structures.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
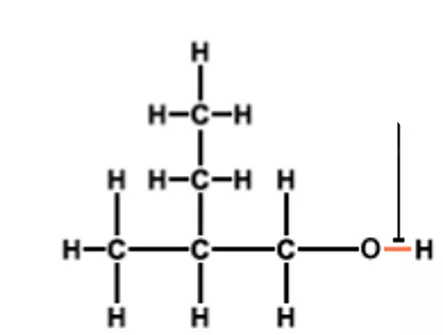
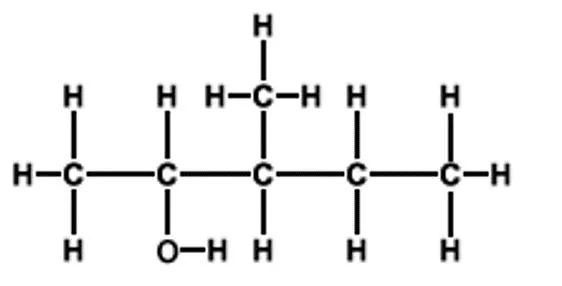
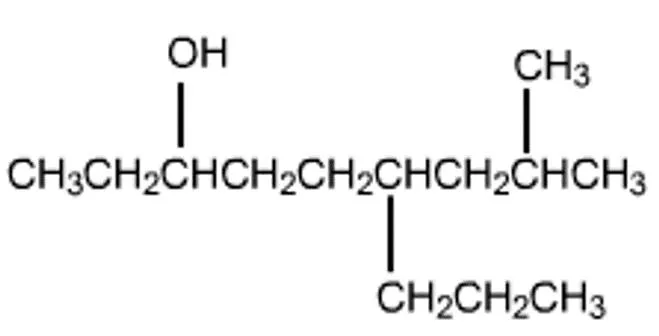
Starter 1. Name these alcohols.
Learning Intention To be able to state the products of the oxidation of alcohols. Success Criteria I can state the products of the oxidation of alcohols I can name the functional group in aldehydes and ketones I can state the difference between aldehydes and ketones
Think Turn your jotter length ways. Draw the following down the left hand side of your jotter leaving space between them. 1. butan-1-ol 2. butan-2-ol 3. 2-methyl propan-2-ol
Classification of alcohols H H CH3 H CH3 H C C H CH3 C C CH3 H3C O H H OH H O Label your alcohols as primary, secondary or tertiary Secondary alcohol, TWOC s joined to the C bonded to the OH group Tertiary alcohol, THREEC s joined to the C bonded to the OH group Primary alcohol, ONEC joined to the C bonded to the OH group H H H CH3 H C C C H H3C C CH3 H OH H OH propan-2-ol 2-methylpropan-2-ol
Primary Alcohol Secondary Alcohol Tertiary Alcohol
Oxidation Oxidation is when the oxygen to hydrogen ratio increases. For this to occur the H in the hydroxyl group is removed. Also the H attached to the C that the hydroxyl is attached to is removed. Which of the previous alcohols will be oxidised? Draw the products of the oxidation reactions.
Experiment 1. Boil some water in a kettle and pour it into the large beaker. 2. Add a 2 cm depth of primary alcohol to one test tube, a 2 cm depth of secondary alcohol to a second test tube and a 2 cm depth of tertiary alcohol to a third test tube. 3. Add acidified potassium dichromate to each of the three test tubes to give a distinct orange colour. 4. Place the test tubes in the hot water bath. 5. Note any colour changes which would indicate that oxidation has taken place.
Oxidation of Alcohols Primary alcohols can be oxidised by a number of oxidising agents, in two stages, 1stStage - Hydrogen is lost. When applied to carbon compounds, oxidation results in an increase in the oxygen to hydrogen ratio. 1st H O oxidation Label the aldehyde and ketone and show that tertiary alcohols R C H + O R C H + H2O aldehyde O H 1st R1 O oxidation R C H + O + H2O R C R1 ketone O H Secondary alcohols can be oxidised to form ketones, Tertiary alcohols do not undergo oxidation.
Primary Alcohol Aldehyde Ketone Secondary Alcohol Tertiary Alcohol
Oxidation of alcohols Primary and secondary alcohols can be oxidised by Acidified Potassium Dichromate orange Cr2O72-(aq) to green Cr3(aq) Copper oxide black to brown Add these oxidising agents to your diagram.
Acidified potassium dichromate orange green Primary Alcohol Aldehyde Copper oxide black brown Ketone Secondary Alcohol Tertiary Alcohol
H OH H H O H H C C C H H C C C H H propan-2-ol H H H H propanone H:O ratio = 6:1 H:O ratio = 8:1 H H H H H C C OH H C C O H H H ethanal ethanol
When primary and secondary alcohols are oxidised, 2H atoms are removed: 1 from the OH and one from the adjacent C. A tertiary alcohol cannot be oxidised as it doesn t have any Hs on the adjacent C atom. CH3 no Hs attached H3 C C CH3 OH
Think Oxidation can happen again to either the aldehyde or the ketone. Which one do you think will go through oxidation? How can this be achieved? (Think about the definition for oxidation.)
Oxidation of Alcohols 2nd Stage - oxygen is gained. When applied to carbon compounds, oxidation results in an increase in the oxygen to hydrogen ratio. 1st O O oxidation R C H + O R C H Carboxylic acid O 2nd R C R1 ketone
Oxidation INTRODUCTION Both aldehydes and ketones contain the carbonyl group. C O In aldehydes a hydrogen atom is bonded to the carbonyl group but in ketones the carbonyl group is always flanked by carbon atoms: O O C H C C C aldehyde ketone This structural difference accounts for the fact that aldehydes can undergo mild oxidation to form carboxylic acids but ketones resist oxidation. Oxidising agents can therefore be used to distinguish between aldehydes and ketones. The aim of this experiment is to use the mild oxidising agents, acidified potassium dichromate solution, Benedict's solution and Tollens' reagent, to distinguish between two given carbonyl compounds one of which is an aldehyde and the other a ketone.
Oxidation Procedure 1. Boil some water and place it into a large beaker. 2. Add sulphuric acid to each of two test tubes to a depth of about 2 cm. Then add potassium dichromate solution to both to give a total depth of about 3 cm in each. 3. To one of these test tubes add about 5 drops of compound X and to the other add about 5 drops of compound Y. 4.Place both test tubes in the water bath and observe and record any changes. 5.Add Fehling s reagent to each of two test tubes to a depth of about 3 cm. 6. Repeat steps 3 and 4. 7. Add Tollens' reagent to each of two veryclean test tubes to a depth of about 3 cm. 8. Repeat steps 3 and 4 and immediately after, wash the contents of the test tubes down the drain with large amounts of water.
Oxidation of Alcohols Primary alcohols can be oxidised by a number of oxidising agents, in two stages, 1stStage - Hydrogen is lost; 2nd Stage - oxygen is gained. When applied to carbon compounds, oxidation results in an increase in the oxygen to hydrogen ratio. 1st H O oxidation R C H + O R C H + H2O aldehyde O H 2nd O O oxidation + O R C O H R C H O aldehyde Carboxylic acid R C R1 Secondary alcohols can be oxidised to form ketones, Tertiary alcohols do not undergo oxidation. ketone
Aldehydes and Ketones Distinguishing tests (Using mild oxidising agents.) Aldehydes are oxidised to carboxylic acids Ketones do not react with mild oxidising agents 1. Fehlings solution contains Cu2+ ions (blue) which form Cu+ ion (orange-red) in the presence of aldehydes. Cu2+ + e- Cu+ 2. Tollen s reagent (silver mirror test) (colourless to silver mirror) Ag+ + e- Ag 3. Acidified Potassium Dichromate orange Cr2O72-(aq) to green Cr3(aq) Cr2O72-+ 14H+ 6e- 2Cr3+ + 7H2O Write the ion electron equations for the reduction of the oxidising agents
H H H OH H C C H C C O O H H ethanoic acid ethanal O:H 4:1 O:H 4:2 = 2:1 OH C O
Everything you need to know about the oxidation of alcohols!!! Acidified potassium dichromate orange green Carboxylic acid Primary Alcohol Aldehyde Acidified potassium dichromate orange green Copper oxide black brown Benedict s solution blue orange/red Ketone Secondary Alcohol Tollen s reagent colourless silver Tertiary Alcohol
Oxidation of Alcohols Primary Alcohol Aldehyde Carboxylic acid O O R O H C O H C H R R Secondary alcohol Ketone H O O R C R1 R C R1 Tertiary alcohol does not undergo oxidation OH R C R1 R2
Naming alcohols, aldehydes and ketones Information from BBC Bitesize on the rules for naming organic compounds. Oxidising reagents Information on the colour changes for different oxidising agents
Functional Groups The alcohols all contains the hydroxyl group. The carboxylic acids all contains the carboxyl group. The alkanals and alkanones all contains the carbonyl group.