Organic Chemistry: Functional Groups and Naming Rules
Delve into the world of organic chemistry with a focus on functional groups like alcohols, ethers, aldehydes, and ketones. Explore the rules for naming these compounds, understanding their structures, and how they impact the properties of molecules. From alcohols with hydroxy groups to ketones containing carbonyl groups, unravel the intricacies of organic compounds in this comprehensive overview.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Organic Chemistry Overview: Packet #2 (yellow) (we got this!)
Functional Groups = special add-ons that contribute changes to the structure which contribute changes to the properties and functions of the overall molecule!
Alcohols Alkyl group is attached to a hydroxy group (-OH) = the functional group Basically the same rules as naming alkanes & alkenes, with a few additions
The Rules: 1. Find the longest chain of carbons... it must contain the OH group! 2. # the chain to give the OH bonded carbon the lowest possible number 3. Make sure that # shows up in the name! 4. Add the ol ending to show it s an alcohol! METHANOL ETHANOL 1 - PROPANOL ISOPROPANOL [These are Common Names (boo) we want IUPAC!]
Ethers Organic compound that contain a C-O-C arrangement within the chain Basically: there will be an alkyl group on each side of a central oxygen ethoxyethane
The Rules 1. The alkyl groups here are called alkoxy groups... They get an oxy ending! 2. If the parent chain = 3+ carbons, use a # to indicate the position of the alkoxy group. Parent Chain = ethane (they re equal here so it doesn t really matter which side is which) Alkoxy group = ETHOXY (2 carbons long + -oxy ending) Why no number in the name?
# drama If the parent chain has 3+ carbons, there needs to be a number in the name... It should correspond to the # carbon the alkoxy group is coming off of (see below)
Aldehydes & Ketones Contain a carbonyl group (C=O) Aldehyde: C=O with 1+ H attached to the C the carbonyl carbon is ALWAYS a terminal carbon Ketone: C=O with 2 C s attached to the central C the carbonyl carbon is NEVER a terminal carbon
Rules for Aldehydes 1. Identify the parent compound 2. The carbonyl carbon = carbon #1 (so there s no need to # the functional group here!) 3. Add the al ending
Rules for Ketones 1. Identify the parent compound 2. # the chain so the carbonyl carbon gets the lowest number possible 3. Add the one ending
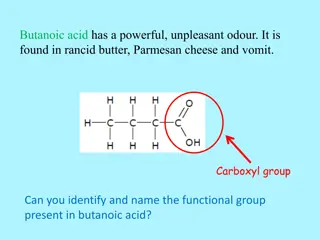
Organic Acids Contain a carboxyl group (a.k.a. carboxylic acid group) NOT AN ALCOHOL, ALDEHYDE, OR KETONE! It s a separate thing! This functional group always occurs on a terminal carbon of the parent (so no # needed in the name)
Rules for Organic Acids 1. Identify the parent compound 2. The carboxyl carbon = carbon #1 (so there s no need to # the functional group here!) 3. Add the oic ending + acid
Esters Can be considered derivatives of organic acids... Except the hydrogen atom on the hydroxy group is replaced with an organic group such as an alkyl group (represented by R) They usually smell yummy!!!
Rules for Esters Identify the parent compound Determine the name of the R-group Place the name of the R-group in front of the name of the parent acid (2 words!) Add the ate ending Make sure to eliminate the acid part of the name!
Amines Organic compounds related to ammonia (NH3) They have NITROGEN in them! Primary Amine: 1 N, 2 H s (replaced by 1 alkyl) Secondary Amine: 1 N, 1 H (replaced by 2 alkyls) Tertiary Amine: 1 N, no H s (replaced by 3 alkyls)
Rules for Amines Secondary/Tertiary Amines: 1. named by adding the names(s) of the alkyl group(s) attached to the N to the word amine In ABC order Ex: ethylisopropylmethylamine Primary Amines: 1. Treat the NH2 group (a.k.a. amino group) like a substituent on the longest chain of carbons Examples: * Aminomethane These dastardly so- and-so s can have Common Names! (boo) * 3-aminohexane
Amides Compounds very similar to organic acids, except instead of a hydroxy group attached to the C=O, it s an amino group! Woo! The functional group is sometimes written as CONH2 to save room! The good news? We ll only look at Primary Amides (although there are others... Ugh)
Rules for Amides 1. Identify the organic acid from which the amide was derived and change the oic ending to amide AND drop the acid part 2. Add the names of any alkyl groups --- should be a one word name!
Halogenated Hydrocarbons Oftentimes synthetic, these are basically just hydrocarbons with F, Cl, Br, and/or I (the halogens) acting as functional groups These things are ridonculously important to Organic Chem... Name them now, but learn the crazy mechanisms for creating them in college!
Rules for H-Hs 1. Drop the ine suffix from the name of the halogen atom(s) and add a suffix of o 2. Add the altered name(s) to that of the parent compound 3. Make sure to use #s to indicate where the halogens show up on the parent compound (if there are more than 2 C s) *** if multiple halogens show up, you have to use # s even on a methane or ethane parent chain!