Canizzaro Reaction in Organic Chemistry: Experiment and Applications
The Canizzaro reaction involves the disproportionation of aldehydes in the presence of a strong base to produce an alcohol and a carboxylic acid. This experiment, supervised by Lecturer Israa Radhi, explores the mechanism and practical application of the reaction. Benzyl alcohol and benzoic acid, pr
1 views • 7 slides
Aldol Condensation Reaction: Preparation of Chalcones
Chalcones are important unsaturated aromatic ketones that serve as biogenetic precursors of flavonoids and isoflavonoids. They have various medicinal and pharmaceutical applications due to their biological activities. Chalcones are easily synthesized compounds with potential therapeutic uses, making
2 views • 13 slides
Understanding the Seliwanoff Color Reaction and its Significance
The Seliwanoff color reaction, discovered by Russian chemist Feodor Feodorovich Selivanov, is used to differentiate between aldoses and ketohexoses based on their dehydration and reaction with resorcinol in acidic conditions. Ketoses like fructose react faster than aldoses like glucose, leading to a
3 views • 20 slides
Understanding the Diels-Alder Reaction in Practical Organic Chemistry
The Diels-Alder reaction is a fundamental method in organic chemistry for producing cyclic organic compounds by combining a conjugated diene with an alkene. This reaction, named after Otto Diels and Kurt Alder, involves the formation of a six-membered ring with specific bond rearrangements. Conjugat
4 views • 15 slides
Chemical Kinetics: Understanding Reaction Rates and Factors
Chemical kinetics is a branch of physical chemistry that explores the velocity and factors influencing chemical reactions. It studies how reactants transform into products, considering conditions like temperature, pressure, and reactant concentrations. Factors affecting reaction rates include the na
7 views • 24 slides
Cannizzaro Reaction
The Cannizzaro reaction is a chemical reaction involving the base-induced disproportionation of non-enolizable aldehydes to form a primary alcohol and a carboxylic acid. Discover more about this reaction, its history, mechanism, and variants like the Cross Cannizzaro reaction and Intramolecular Cann
1 views • 20 slides
Benzoin Condensation: A Name Reaction Explained by Dr. Atul Kumar Singh
Benzoin condensation is a classic organic reaction where aromatic aldehydes self-condense to form α-hydroxy ketones. Dr. Atul Kumar Singh, an Assistant Professor of Chemistry, details the mechanism and the specific catalytic properties of cyanide in this reaction. The reaction involves refluxing th
0 views • 6 slides
Investigating Impact of Practice on Human Reaction Time Through Ruler Drop Test
This practical investigation focuses on determining if practice can reduce human reaction times by conducting a ruler drop test. Participants use their weaker hand to catch a ruler dropped by their partner, aiming to improve their reaction time with practice. The experiment explores how athletes can
0 views • 7 slides
Understanding Antigen-Antibody Precipitation Reaction in Microbiology
Antigen-antibody precipitation reaction involves the formation of insoluble products when a soluble bivalent antibody interacts with a soluble antigen. This reaction leads to the formation of a visible precipitate known as a lattice. The mechanism of precipitation, including the prozone phenomenon,
0 views • 20 slides
Kinetic Reaction of Sulphite and Iodate - Landolt Reaction Overview
The kinetic reaction of sulphite ions and iodate in the Landolt reaction is a fascinating chemical process where slow and fast reactions occur sequentially, resulting in a visually striking color change. By monitoring the induction period between the two reactions, one can observe the formation of h
0 views • 9 slides
Understanding Electrochemical Processes in Materials Engineering
Electrochemical processes play a crucial role in materials engineering, specifically in the context of corrosion. These processes involve both oxidation (anodic reaction) and reduction (cathodic reaction) reactions occurring simultaneously. Maintaining a balance between these reactions is essential
3 views • 22 slides
Understanding Chemical Kinetics: Rates, Reactions, and Mechanisms
Chemical kinetics involves studying reaction rates, rate laws, stoichiometry, and factors affecting reaction speed. This branch of chemistry delves into determining reaction orders, rate constants, and activation energies using various methods. Different types of rates, such as initial, instantaneou
2 views • 68 slides
Understanding the Hell-Volhard-Zelinsky Reaction Mechanism
The Hell-Volhard-Zelinsky (HVZ) reaction is a unique halogenation method for carboxylic acids at the alpha carbon, involving phosphorus tribromide and bromine. This mechanism, named after its chemists, requires severe conditions and can lead to specific products or limitations such as beta unsaturat
0 views • 8 slides
Understanding Hammett Parameters in Organic Chemistry
The Hammett Parameters analysis, particularly the Hammett Plot, is a valuable tool in studying the electronic effects of substituents on aromatic systems. This linear free-energy relationship approach aids in optimizing reaction conditions and probing reaction mechanisms. Applications of Hammett Par
0 views • 8 slides
Understanding the Prins-Pinacol Reaction in Organic Chemistry
The Prins-Pinacol reaction involves a two-step process starting with the Prins reaction and followed by the Pinacol rearrangement. This reaction, discovered in 1919 by Hendrick J. Prins, is a crucial transformation in organic chemistry, leading to the formation of important carbonyl compounds. The m
0 views • 14 slides
Understanding Kinetics and Reaction Rates in Chemistry
Kinetics is the study of reaction rates and factors affecting them, such as concentration, temperature, catalysts, and more. Orders of reaction classify reactions based on rate dependency on reactant concentration. Factors like pH, light, and solvents can also impact reaction rates. Half-life and sh
0 views • 18 slides
Aldol Condensation Reaction for Benzalacetophenone Preparation
Aldol condensation is a key reaction for preparing benzalacetophenone, also known as chalcones. Chalcones are unsaturated aromatic ketones with various medicinal applications, showcasing activities like anti-diabetic, anti-inflammatory, and anti-bacterial effects. The reaction involves combining ben
0 views • 10 slides
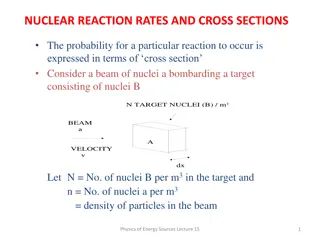
Understanding Nuclear Reaction Rates and Cross Sections
Nuclear reaction rates and cross sections play a crucial role in determining the probability of interactions between particle beams and target nuclei. Cross section is the effective area of a target nucleon to the incident beam, with the interaction probability calculated based on the number density
1 views • 13 slides
Understanding Chemical Kinetics: Reaction Rates and Mechanisms
Chemical kinetics is a branch of chemistry focused on studying reaction rates and mechanisms. Unlike thermodynamics, which deals with feasibility, kinetics explores the speed at which reactions occur. Factors such as temperature, pressure, and catalysts influence reaction rates. Understanding the ra
3 views • 72 slides
Baeyer-Villiger Oxidation: Mechanism and Migration Aptitude
The Baeyer-Villiger oxidation is a key organic reaction that transforms ketones into esters or cyclic ketones into lactones using peroxyacids. The mechanism involves protonation of the carbonyl group and migration of substituents. Migration aptitude in this reaction determines the regioselectivity,
0 views • 10 slides
Understanding Reaction Rates in Chemistry: Apparatus, Experiment, and Analysis
Explore the concept of reaction rates in chemistry through the use of a gas syringe apparatus, conducting experiments, analyzing results, and understanding factors affecting reaction rates. Dive into hands-on activities and graphical representations to enhance your understanding of this fundamental
0 views • 16 slides
Sprinting Event - Identifying Gold, Silver, and Bronze Medallists
In a sprinting event, the reaction time between the gun firing and athletes leaving the starting block is crucial. This task involves identifying the top three medallists based on their lane number, reaction time, and final time in a 100-meter sprint race. By analyzing the data of 8 runners provided
0 views • 10 slides
Exploring Reaction-Diffusion Systems and Random Walks in Chemistry
Delve into the fascinating world of reaction-diffusion systems and random walks in chemistry, exploring concepts such as well-mixed reactive systems, diffusion-reaction dynamics, finite differences, and incorporating reactions into random walks. Discover how these principles play a crucial role in u
0 views • 29 slides
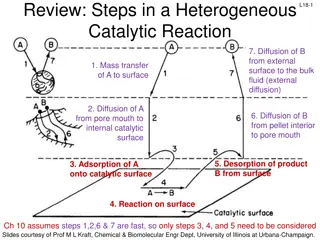
Understanding Heterogeneous Catalytic Reactions: Key Steps Explained
In a heterogeneous catalytic reaction, various important steps occur, including diffusion of reactants, adsorption onto the catalyst surface, surface reactions, and desorption of products. Different mechanisms like single-site, dual-site, and Eley-Rideal mechanisms are involved in the surface reacti
0 views • 17 slides
Utilizing a Global Model for Analyzing Reaction Pathways in Plasma Systems
This research focuses on using a kinetic global model framework to identify relevant reactions in chemically complex plasma systems. The framework, KGMf, enables the investigation of macroscopic plasma characteristics by analyzing reaction pathways, sensitivity to reaction rate errors, and dominant
1 views • 6 slides
Understanding Chemical Reaction Kinetics in Chemcad
Explore the kinetic reactor module in Chemcad for reaction rate specification using VBA. Learn how to determine parameter values and analyze reactions such as steam reforming and water gas shift. Follow step-by-step instructions to run the unit operation, view reactor profiles, and compare reaction
0 views • 9 slides
Understanding Chemical Reaction Kinetics: From Unimolecular to Three-Body Reactions
Explore the fundamental concepts of chemical reactions, including unimolecular reactions like thermolysis and photolysis, bimolecular reactions, and three-body reactions. Learn about rate constants, reaction mechanisms, and the impact of pressure on reaction rates. Discover how energy transfer, phot
0 views • 9 slides
Understanding Free Energy, Reaction Quotient, and Equilibrium Constant
This educational material delves into the concepts of free energy, reaction quotients, and equilibrium constants in chemical systems. It explains how to determine the direction of a reaction based on Q and K values, elucidates the role of Gibbs free energy in determining spontaneity, and provides ca
0 views • 10 slides
Implicit vs. Explicit Processing in L2 Learning and Reaction Times
In second language research, the focus on implicit versus explicit processing is crucial for theory and pedagogy. Research by Batterink et al. (2014) explored L2 learning without awareness, revealing varied effects on reaction times and ERP responses based on participants' awareness of hidden gramma
0 views • 28 slides
Grignard Reaction in Chemistry Lab: Part 1 Overview
The Grignard Reaction Part 1 in Chemistry 318 Fall 2018 involves the preparation of the Grignard reagent, its reaction with CO2, and the isolation of the benzoic acid product. The experiment spans two lab sessions, focusing on safety precautions, pre-lab checks, and upcoming due dates. Students are
0 views • 11 slides
Introduction to Chemical Reaction Engineering (CRE)
Chemical Reaction Engineering (CRE) focuses on studying the rates and mechanisms of chemical reactions, as well as designing reactors for these reactions. The field involves understanding balances in terms of molar flow rates, mole balances, rate laws, stoichiometry, and membrane reactors. Membrane
0 views • 20 slides
Understanding Chemical Kinetics: The Rate of Reaction and Equilibria
Chemical kinetics explores the rate at which chemical reactions occur and the factors influencing them. This tutorial delves into the concepts of reaction rates, equilibrium, collision theory, and the role of concentration in determining reaction rates. By understanding these principles, industries
0 views • 117 slides
Understanding Antigen-Antibody Precipitation Reaction in Immunity
The humoral basis of immunity involves the specific reaction between antigens and antibodies, resulting in the formation of insoluble precipitates through a process called precipitation. This reaction plays a crucial role in immune responses against infectious diseases, influenced by factors like af
0 views • 16 slides
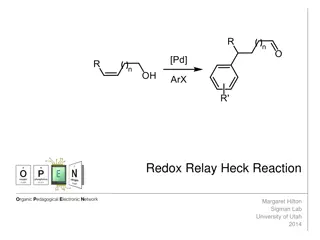
Understanding the Redox-Relay Heck Reaction in Organic Synthesis
The Redox-Relay Heck Reaction is a powerful tool in organic synthesis that allows for the functionalization of olefins with aryl groups. Developed by Sigman and colleagues, this reaction involves a palladium-catalyzed relay controlled by a thermodynamic sink, leading to the formation of aldehydes or
0 views • 6 slides
Understanding Chemical Kinetics: Reaction Rates and Activation Energy
Exploring the fundamental concepts of chemical kinetics, this content delves into reaction rates, collision theory, and activation energy in chemical reactions. It emphasizes the importance of particle collisions, correct orientation, and energy requirements for reactions to occur. Through energy di
0 views • 17 slides
Organic Chemistry: Aldol Condensation Experiment Overview
Organic chemistry students learn about the Aldol condensation reaction involving ketones and aldehydes. The experiment involves the reaction of acetone with benzaldehyde catalyzed by sodium hydroxide to form a trans, trans-isomer. The reaction is illustrated step by step, from the formation of the e
0 views • 11 slides
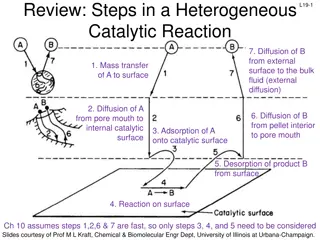
Understanding Heterogeneous Catalytic Reactions: Steps and Mechanisms
The content discusses the key steps involved in a heterogeneous catalytic reaction, focusing on diffusion, mass transfer, adsorption, desorption, and surface reactions. It highlights the importance of considering external diffusion effects and provides guidelines for deducing reaction mechanisms in
0 views • 40 slides
Synthesis of Banana Oil in Chemistry Class
The synthesis of banana oil involves a reaction between carboxylic acid and alcohol catalyzed by H2SO4 to produce isoamyl acetate. The mechanism includes nucleophilic acyl substitution with two main reaction steps - addition and elimination. The process shifts the equilibrium to favor product format
0 views • 16 slides
Understanding Kinetics in Chemical Reactions
Kinetics is the study of reaction rates and factors affecting them. Reaction rate is the speed at which a reaction occurs, influenced by factors like concentration, temperature, pH, light, catalysts, and solvents. Reactant concentration determines reaction order, which categorizes reactions as zero-
0 views • 18 slides
Understanding Skill-Related Fitness: Physical and Emotional Aspects
Skill-related fitness encompasses both physical and emotional components, such as agility, balance, coordination, power, reaction time, and speed. Physical agility involves quick body movements, while emotional agility relates to positive responses in various situations. Physical balance requires we
0 views • 7 slides